(Nanowerk Spotlight) Much attention of nanotechnology researchers has recently been paid to the fabrication of free-standing, ultra-thin films. These systems have been developed for use in a wide variety of fields such as nano-separation membranes or nanosensors for electrochemical and photochemical applications. In a first report on the fabrication of free-standing nanosheets for biomedical applications, scientists in Japan have developed a biodegradable thin film of only about 20 nanometers thickness that could replace surgical stitches.
"We have developed a free-standing PLLA nanosheet with a thickness of 20nm and an area in centimeter order, fabricated by a simple combination of a spin-coating method and a peeling technique with PVA as a supporting film,"
Shinji Takeoka tells Nanowerk. "We found that our ultra-thin nanosheet has an excellent sealing efficacy for gastric incision as a novel wound dressing that does not require adhesive agents. Furthermore, the sealing operation repaired the incision completely without scars and tissue adhesion. This approach would constitute an ideal candidate for an alternative to conventional suture/ligation procedures, from the perspective not only of a minimally invasive surgical technique but also reduction of operation times."Takeoka is a professor in the Department of Department of Life Science and Medical Bioscience at Waseda University in Tokyo. With collaborators from the National Defense Medical College and members of his group he reports his findings in a recent paper in "Free-Standing Biodegradable Poly(lactic acid) Nanosheet for Sealing Operations in Surgery"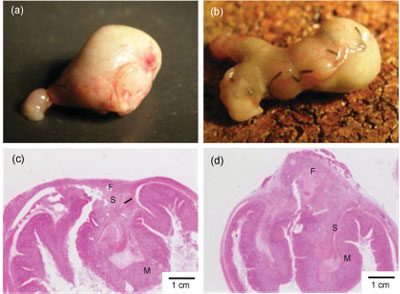
| Macroscopic and microscopic observations of the stomach seven days after treatments. Sealing with the PLLA nanosheet (a,c). Conventional suture/ligation (b,d). Letters F, M, and S show fibroblasts, mucosa, and submucosa, respectively. |
Sealing surgical incisions with this nanosheet instead of performing the traditional suture and ligation procedures is more than just a godsend for Hollywood's plastic surgeons. In cases where internal organs or other tissue has been severely damaged, repair by suture and ligation may become technically difficult and unreliable. Also, internal scarring can lead to sometimes dangerous adhesions, with areas of tissue that should remain separate become joined by scar tissue.Researchers have already been working on novel glue-type (see for instance: Gel-based glue fastens snails to wet surfaces, model for surgical adhesive and sheet-type biomaterials for wound dressing. "Ideally" explains Takeoka, "desirable properties of a novel wound dressing material should incorporate the advantages of both types, that is, not only sufficient adhesive strength to cover the wound site as with a sheet-type structure, but also compatibility with the site as with a glue-type structure."The nanosheet developed by the Japanese team fits that bill – it has very interesting features such as high adhesiveness, high flexibility, and high transparency at the same time. PLLA and other polyesters have been clinically applied in drug delivery and as degradable stitches before, but not as nano-thin sheets. Interestingly, the mechanical properties of the nanosheets are very different from the conventional thin film of the same components.
t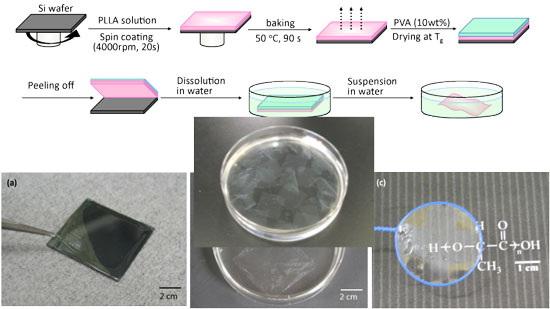 |
Preparation of PLLA nanosheets and appearance. (Image: Dr. Shinji Takeoka, Waseda University)
|
| Takeoka explains that his and his team's scientific backgrounds are bio-related macromolecular chemistry and molecular assembling science and engineering." We have studied platelet substitutes, which are biodegradable nanoparticles such as phospholipid vesicles and polymerized albumin particles carrying platelet membrane proteins and peptides for ten years" he says. "We noticed that the nanoparticles specifically accumulated at sites of vascular injury and had hemostatic ability. Moreover, in order to enhance their hemostatic abilities, we proposed the idea of sheet-shaped carriers having a larger contact area for a targeting site than spherical nanoparticles." To demonstrate the suitability of their nanosheets as a novel wound dressing material that lacks adhesive reagents, the researchers repaired incisions on mouse stomachs with them instead of sutures. They found that the PLLA nanosheet has an excellent sealing efficacy and the healed wound showed neither scar nor tissue adhesion. Although it will be several years before this material is developed enough to enter clinical trials, Takeoka expects that the nanosheet will be applied not only in the field of surgery as a wound dressing instead of conventional suturing operation but also in the field of plastic surgery, endoscopic surgery, regeneration medicine, and external use (skin etc.). He also sees applications in the fields of material science, material engineering, and surface science. |





.jpg)



