Stefanie Schulz-Schupke, M.D., of the Deutsches Herzzentrum Munchen, Technische Universitat, Munich, Germany and colleagues assessed whether vascular closure devices are noninferior (not worse than) to manual compression in terms of access site-related vascular complications in patients undergoing diagnostic coronary angiography. The study appears in the November 19 issue of JAMA, a cardiovascular disease theme issue.
Percutaneous (through the skin) coronary angiography and interventions have become a cornerstone in the diagnosis and treatment of coronary artery disease. A substantial proportion of the adverse effects associated with these procedures is related to access-site complications. The common femoral artery (a large artery in the groin) is still the most frequently used access site. After the procedure, closure of the artery access site is usually achieved by manual compression. Since the mid-1990s, however, vascular closure devices (VCDs) have been introduced into clinical practice with the aim of improving efficacy and safety. Different types of VCDs have been developed, including intravascular and extravascular. However, concern exists about the safety of VCDs in comparison with manual compression, according to background information in the article.
For this study, conducted at four centers in Germany, 4,524 patients undergoing coronary angiography via the common femoral artery were randomly assigned to receive an intravascular VCD (n = 1,509), extravascular VCD (n = 1,506), or manual compression (n = 1,509) to achieve hemostasis (defined as no bleeding or only light superficial bleeding and no expanding hematoma [a localized swelling filled with blood]). Before hospital discharge, imaging of the access site was performed in 4,231 (94 percent) patients.
The primary end point (the composite of access site-related vascular complications at 30 days after randomization with a two percent noninferiority margin) was observed in 208 patients (6.9 percent) assigned to receive a VCD and 119 patients (7.9 percent) assigned to manual compression (difference, -1.0 percent). In addition, the time to hemostasis was significantly shorter with VCD compared with manual compression; time to hemostasis was shorter with intravascular VCD vs extravascular VCD; and device failures were less frequent with intravascular VCD vs extravascular VCD.
The authors write that the results of this trial may represent an important development for the clinical use of these devices. "Overall, the increase in efficacy of VCD use, with no trade-off in safety, provides a sound rationale for the use of VCD over manual compression in daily routine."
Sunday, November 16, 2014
Comparison of methods to achieve artery closure following coronary angiography
Labels:
vascular closure
Start-up pitches high-tech glue for surgical leaks
TEL AVIV — An Israeli medical-device start-up is tackling one of the most dangerous occurrences in surgery — and it's doing it with glue.
LifeSeal is a glue-like substance that augments and, in some surgeries like hernias, replaces the traditional and painful sealing procedures of staples, tacks and sutures.
The privately-owned Israeli company behind the high-tech glue, LifeBond, says it should help in the treatment of post-operative leaks in closures of gastrointestinal and other surgical wounds. Patients get back up to speed more quickly and are more comfortable as they do.
Orahn Preiss-Bloom, one of LifeBond's co-founders, says the company's proprietary materials, which combine gelatin and an enzyme and are delivered by an applicator, were inspired by two sources: research by Professor Gregory Payne at the University of Maryland and the use of the enzyme for food applications in Asia.
The company is backed by some of Israel's top venture-capital firms as well as by Robert Taub, a prominent medical-innovation investor. And the idea is strong enough that Johnson & Johnson (TICKER: JNJ), the New Jersey-based international health-care giant, has put money down on LifeBond.
The technology is currently in European clinical trials. And with an eye to entering the U.S. market as well, CEO Gideon Sturlesi and Preiss-Bloom are looking for partners and raising capital.
TWO CURRENT APPLICATIONS
Started in 2007 and employing 35 people from headquarters in Caesarea, Israel, LifeBond for now is focusing the technology's application on gastrointestinal surgery and bariatric weight-loss procedures as well as in hernia surgery.
Colon-cancer surgeries require what the doctors call anastomosis, removal of diseased intestine and reattachment to restore the gastronintestinal tract's functionality. The further down in the colon a surgeon must work, the higher the risk that a seal will leak, Sturlesi says. In some 15% to 25% of lower-colon operations, the seals leak, exposing the patients to infection and additional surgery, even death.
In such procedures, after a surgeon applies staples to close the colon, he or she spreads LifeSeal – using a glue-like applicator – along the line of the closure. The sealant provides a secure and elastic barrier to infection while the body heals. Tissue grows in and the sealant gradually dissipates.
LifeBond's second current major application, which the company calls LifeMesh, targets another very common surgery: hernias, the breaks in people's abdominal walls.
To secure a protruding intestine back into the abdomen, surgeons usually tack a mesh into place to close the break. These tacks cause inflammation and pain.
Here, the same proprietary material used in LifeSeal becomes an adhesive. In both open hernia surgery and procedures with a laparascope, the surgeon coats a standard mesh patch with LifeSeal and places it to secure the abdominal wall. The surgeon can reposition the mesh if and when necessary. LifeBond says the product keeps the patch in place as the body's tissues grow in and then dissipates.
LifeBond's two co-founders are Preiss-Bloom, 32, a New Yorker who is chief technology officer; and Ishay Attar, 42, who was vice president of business development at what is now Trendlines Medical, a tech-firm incubator. Both have master's degrees in biomedical engineering from the Technion, the Israel Institute of Technology. Attar remains a shareholder but doesn't hold a position with LifeBond.
CEO Sturlesi, 53, joined the company two years ago. Previously he was executive vice president at Lumenis, the producer of laser equipment for medical and cosmetic applications, and he was a co-founder of Galil Medical, a producer of cryosurgical technology. LifeBond's chairman is Ittai Harel, general partner of Pitango, a Herzliya, Israel, venture-capital firm and investor in LifeBond.
Worldwide market: $450 mln
LifeBond's first two target markets are substantial. The company estimates that annually in the U.S., 300,000 colorectal anastomosis procedures are done each year. The company pegs the worldwide market for this application at $450 million, a third of it in the U.S. And LifeBond says 2 million hernia repairs are done annually worldwide, half of them in the U.S. That's overall also a $450 million market.
LifeBond's investors include the Israeli venture-capital firms Aurum, Giza and Pitango. Also an investor, and a director, is Robert Taub, who founded Omrix, a producer of sealant used to control bleeding during surgery. J&J acquired Omrix in 2007.
In 2011, when LifeBond raised $20 million in a third-round financing, the New Brunswick, N.J., health-care giant J&J joined the round. Its specific investment hasn't been disclosed. LifeBond previously raised $1.5 million and $8.5 million in its Series A and Series B rounds respectively.
LifeBond is now in a Series D round, aiming to raise a total of $25 million by early 2015, and current investors have committed about half that figure.
In first-half 2013, LifeBond did a first clinical study in Sweden to test the application method for LifeSeal and to evaluate the product for safety. That study met its goals and this year LifeBond enrolled patients in a second study at eight centers in Belgium, Israel and Sweden. The company is expecting the results of that trial in November. The study's goal is to receive the CE Mark, which means that the product meets EU standards.
Sturlesi says the company has started the U.S. Food and Drug Administration process to develop the studies it needs to gain clearance to market LifeSeal in the U.S.
The company sees additional applications for LifeBond's system in surgeries involving the eye, brain, lungs, spine, urological system, and ear, nose and throat.
"We are there to support the natural healing process of the body," Sturlesi says.
LifeSeal is a glue-like substance that augments and, in some surgeries like hernias, replaces the traditional and painful sealing procedures of staples, tacks and sutures.
The privately-owned Israeli company behind the high-tech glue, LifeBond, says it should help in the treatment of post-operative leaks in closures of gastrointestinal and other surgical wounds. Patients get back up to speed more quickly and are more comfortable as they do.
Orahn Preiss-Bloom, one of LifeBond's co-founders, says the company's proprietary materials, which combine gelatin and an enzyme and are delivered by an applicator, were inspired by two sources: research by Professor Gregory Payne at the University of Maryland and the use of the enzyme for food applications in Asia.
The company is backed by some of Israel's top venture-capital firms as well as by Robert Taub, a prominent medical-innovation investor. And the idea is strong enough that Johnson & Johnson (TICKER: JNJ), the New Jersey-based international health-care giant, has put money down on LifeBond.
The technology is currently in European clinical trials. And with an eye to entering the U.S. market as well, CEO Gideon Sturlesi and Preiss-Bloom are looking for partners and raising capital.
TWO CURRENT APPLICATIONS
Started in 2007 and employing 35 people from headquarters in Caesarea, Israel, LifeBond for now is focusing the technology's application on gastrointestinal surgery and bariatric weight-loss procedures as well as in hernia surgery.
Colon-cancer surgeries require what the doctors call anastomosis, removal of diseased intestine and reattachment to restore the gastronintestinal tract's functionality. The further down in the colon a surgeon must work, the higher the risk that a seal will leak, Sturlesi says. In some 15% to 25% of lower-colon operations, the seals leak, exposing the patients to infection and additional surgery, even death.
In such procedures, after a surgeon applies staples to close the colon, he or she spreads LifeSeal – using a glue-like applicator – along the line of the closure. The sealant provides a secure and elastic barrier to infection while the body heals. Tissue grows in and the sealant gradually dissipates.
LifeBond's second current major application, which the company calls LifeMesh, targets another very common surgery: hernias, the breaks in people's abdominal walls.
To secure a protruding intestine back into the abdomen, surgeons usually tack a mesh into place to close the break. These tacks cause inflammation and pain.
Here, the same proprietary material used in LifeSeal becomes an adhesive. In both open hernia surgery and procedures with a laparascope, the surgeon coats a standard mesh patch with LifeSeal and places it to secure the abdominal wall. The surgeon can reposition the mesh if and when necessary. LifeBond says the product keeps the patch in place as the body's tissues grow in and then dissipates.
LifeBond's two co-founders are Preiss-Bloom, 32, a New Yorker who is chief technology officer; and Ishay Attar, 42, who was vice president of business development at what is now Trendlines Medical, a tech-firm incubator. Both have master's degrees in biomedical engineering from the Technion, the Israel Institute of Technology. Attar remains a shareholder but doesn't hold a position with LifeBond.
CEO Sturlesi, 53, joined the company two years ago. Previously he was executive vice president at Lumenis, the producer of laser equipment for medical and cosmetic applications, and he was a co-founder of Galil Medical, a producer of cryosurgical technology. LifeBond's chairman is Ittai Harel, general partner of Pitango, a Herzliya, Israel, venture-capital firm and investor in LifeBond.
Worldwide market: $450 mln
LifeBond's first two target markets are substantial. The company estimates that annually in the U.S., 300,000 colorectal anastomosis procedures are done each year. The company pegs the worldwide market for this application at $450 million, a third of it in the U.S. And LifeBond says 2 million hernia repairs are done annually worldwide, half of them in the U.S. That's overall also a $450 million market.
LifeBond's investors include the Israeli venture-capital firms Aurum, Giza and Pitango. Also an investor, and a director, is Robert Taub, who founded Omrix, a producer of sealant used to control bleeding during surgery. J&J acquired Omrix in 2007.
In 2011, when LifeBond raised $20 million in a third-round financing, the New Brunswick, N.J., health-care giant J&J joined the round. Its specific investment hasn't been disclosed. LifeBond previously raised $1.5 million and $8.5 million in its Series A and Series B rounds respectively.
LifeBond is now in a Series D round, aiming to raise a total of $25 million by early 2015, and current investors have committed about half that figure.
In first-half 2013, LifeBond did a first clinical study in Sweden to test the application method for LifeSeal and to evaluate the product for safety. That study met its goals and this year LifeBond enrolled patients in a second study at eight centers in Belgium, Israel and Sweden. The company is expecting the results of that trial in November. The study's goal is to receive the CE Mark, which means that the product meets EU standards.
Sturlesi says the company has started the U.S. Food and Drug Administration process to develop the studies it needs to gain clearance to market LifeSeal in the U.S.
The company sees additional applications for LifeBond's system in surgeries involving the eye, brain, lungs, spine, urological system, and ear, nose and throat.
"We are there to support the natural healing process of the body," Sturlesi says.
Synthetic platelets helps clot blood faster
New York: Taking a cue from the human body’s own blood-clotting process, researchers at University of California, Santa Barbara have developed synthetic platelets that can do more than clot blood.
By creating nanoparticles that mimic the shape, flexibility and surface biology of the body’s own platelets, the team was able to accelerate natural healing processes while opening the door to therapies and treatments that can be customised to specific patient needs.
“This is a significant milestone in the development of synthetic platelets, as well as in targeted drug delivery,” said Samir Mitragotri, director of Center for Bioengineering (CBE) who specialises in targeted therapy technologies.
In case of blood loss due to minor injury, platelets release chemicals that “call” other platelets to the site, eventually plugging the wound.
But what happens when the injury is too severe or the patient is on anti-coagulation medication or is otherwise impaired in his or her ability to form a clot, even for a modest or minor injury?
That’s where platelet-like nanoparticles (PLNs) come in.
“These tiny, platelet-shaped particles that behave just like their human counterparts can be added to the blood flow to supply or augment the patient’s own natural platelet supply, stemming the flow of blood and initiating the healing process,” researchers emphasised.
“We were actually able to render a 65 percent decrease in bleeding time compared to no treatment,” said graduate student researcher Aaron Anselmo, lead author of the study.
With PLNs, emergency situations can be brought under control faster, injuries can heal more quickly and patients can recover with fewer complications, he added.
The results appeared in the journal ACS Nano.
By creating nanoparticles that mimic the shape, flexibility and surface biology of the body’s own platelets, the team was able to accelerate natural healing processes while opening the door to therapies and treatments that can be customised to specific patient needs.
“This is a significant milestone in the development of synthetic platelets, as well as in targeted drug delivery,” said Samir Mitragotri, director of Center for Bioengineering (CBE) who specialises in targeted therapy technologies.
In case of blood loss due to minor injury, platelets release chemicals that “call” other platelets to the site, eventually plugging the wound.
But what happens when the injury is too severe or the patient is on anti-coagulation medication or is otherwise impaired in his or her ability to form a clot, even for a modest or minor injury?
That’s where platelet-like nanoparticles (PLNs) come in.
“These tiny, platelet-shaped particles that behave just like their human counterparts can be added to the blood flow to supply or augment the patient’s own natural platelet supply, stemming the flow of blood and initiating the healing process,” researchers emphasised.
“We were actually able to render a 65 percent decrease in bleeding time compared to no treatment,” said graduate student researcher Aaron Anselmo, lead author of the study.
With PLNs, emergency situations can be brought under control faster, injuries can heal more quickly and patients can recover with fewer complications, he added.
The results appeared in the journal ACS Nano.
Labels:
nano-technology
Monday, November 3, 2014
Wednesday, October 8, 2014
HaemoCer approved for use in China and Canada
BioCer Entwicklungs GmbH (BCE), a German implantable biological medical device development and manufacturing company, announced today that it has achieved approval to commence sales of HaemoCer™, an Absorbable Polysaccharide Hemostat (APH), in the People's Republic of China.
"BioCer has now achieved near comprehensive Asia-Pacific regional representation, and the addition of the PRC approval of HaemoCer™ is a significant milestone," Dr Markus Heinlein, Managing Director stated. "The entry into China of our first product HaemoCer™ has been assisted by the early establishment of regional sales representation in the Asia-Pacific region. BCE are pleased Chinese surgeons and patients will now share the benefits of our technology. Our new Health Canada approval is also further evidence of strong international acceptance of our technology."
The PRC approval of HaemoCer™ expands an international sales network encompassing Australia, Hong Kong, India, Korea,Malaysia, New Zealand, Singapore and Thailand within the APEC region. BCE will be exhibiting at the EACTS, Milan, Italy, 11-15thOctober and the CMEF, Chongqing, China, 23-26th October 2014.
Labels:
arista,
Bard,
BioCer,
biocompatible,
Cryolife,
HaemoCer,
medafor,
perclot,
Starch Medical
Tuesday, August 19, 2014
Cardiva Medical, Inc. Announces Completion of the first two tranches of Series 3 Private Equity Financing and the Closing of a Senior Secured Debt Facility with GE Capital
SUNNYVALE, Calif., Aug. 19, 2014 /PRNewswire/ -- Cardiva Medical, Inc. announced today that it has closed the first two tranches of a$16.5 million Series 3 private equity financing and a $12.5 million senior secured facility with GE Capital. The Company will use the proceeds of the Series 3 private equity financing and debt facility to expand commercial efforts for its VASCADE® Vascular Closure System in the United States.
Investing in Series 3 private equity financing was a new investor, Canepa Advanced Healthcare Fund, L.P., who joined existing investors PTV Sciences, AmKey Ventures and TriVentures II Fund L.P. Mr. Paul Enever from Canepa U.S., LLC, which serves as Investment Advisor to Canepa Advanced Healthcare Fund, L.P., has joined the Cardiva Board of Directors.
"We are extremely pleased that Canepa Advanced Healthcare, L.P. has joined Cardiva's current investors in this financing and that Paul Enever has joined our Board of Directors," said Charles Maroney, CEO. Maroney continued, "With the Series 3 equity financing and GE debt facility, Cardiva now has the financial resources to establish VASCADE as a leading extravascular closure technology which we believe can benefit both patients and healthcare providers in the United States by minimizing complications and associated costs and improving patient care when compared to conventional vascular closure devices."
"Cardiva's product platform offers the potential to transform the vascular closure market by improving both patient safety and comfort", said Paul Enever of Canepa U.S., LLC. Enever continued, "VASCADE leverages Cardiva's Catalyst® platform that has been utilized effectively in more than 400,000 procedures since its initial launch into the U.S. market in 2007". We are delighted to add Cardiva to our investment portfolio of emerging growth medical device companies that are focused on improving patient outcomes while reducing the cost of medical care."
Labels:
Cardiva,
Collagen,
vascular closure
Tuesday, August 5, 2014
Transluminal Tech’s velox CD Arterial Closure Device Gets EU Approval

 Transluminal Technologies LLC. out of Syracuse, New York won the CE Mark for its velox CD Vascular Closure Device used to close arteriotomies following percutaneous femoral procedures. Closing arteries can be a challenge, eating up surgeons’ time just when it’s optimal to move the patient out of the OR. The velox CD device speeds up this process by delivering an implant that plugs the vessel wall from within.
Transluminal Technologies LLC. out of Syracuse, New York won the CE Mark for its velox CD Vascular Closure Device used to close arteriotomies following percutaneous femoral procedures. Closing arteries can be a challenge, eating up surgeons’ time just when it’s optimal to move the patient out of the OR. The velox CD device speeds up this process by delivering an implant that plugs the vessel wall from within.
The implant consists of an intraluminal footplate that absorbs within about 24 hours and a extraluminal plug that takes two weeks to absorb. The components are made of a proprietary magnesium alloy that breaks up and bioabsorbs safely into the body, leaving a clean repaired artery without the surgeon having to manually apply pressure during closure.
Here’s a video from a real procedure utilizing the velox CD:
Monday, June 30, 2014
Clot-building nanoparticles raise survival rate following blast trauma Read more: Clot-building nanoparticles raise survival rate following blast trauma
| A type of artificial platelet being developed to help natural blood platelets form clots faster offers promise for saving the lives of soldiers, as well as victims of car crashes and other severe trauma. | |
| In preclinical tests led by a Case Western Reserve University researcher, the artificial platelets, called "hemostatic nanoparticles," when injected after blast trauma dramatically increased survival rates and showed no signs of interfering with healing or causing other complications weeks afterward. | |
| "The nanoparticles have a huge impact on survival—not just in the short term, but in the long term," said Erin Lavik, an associate professor of biomedical engineering at Case Western Reserve. Other researchers had raised concerns that the foreign matter would interfere with healing, or form free-floating clots, but "we saw none of that." | |
| The research, published in the Proceedings of the National Academy of Sciences this week ("Intravenously administered nanoparticles increase survival following blast trauma"), show the survival rate of mice models of blast trauma treated with the nanoparticles increased to 95, compared to 60 percent for those untreated. | |
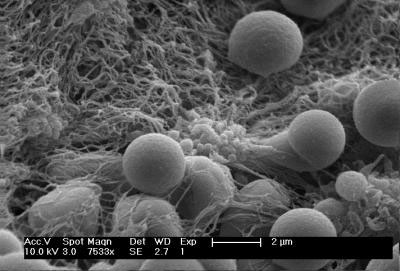 | |
| Spherical hemostatic nanoparticles accumulate on a clot-stabilizing mesh of fibrin the body produces. (Image: Andrew Shoffstall) | |
| Also, no unwanted side effects, such as accumulation of the nanoparticles, clot formation or aberrant healing, were found during examinations one ands three weeks after the injection. | |
| Lavik worked with Margaret M. Lashof-Sullivan, Erin Shoffstall and Kristyn T. Atkins, of Case Western Reserve; Nickolas Keane and Cynthia Bir of Wayne State University and Pamela VandeVord of Virginia Tech. | |
| Explosions account for 79 percent of combat-related injuries and are the leading cause of battlefield deaths, according to researchers at Veterans Affairs hospitals and the federally run Uniformed Services University of the Health Sciences. | |
| The primary blast wave, flying shrapnel and being thrown to the ground cause the lungs, liver, kidneys and other organs to hemorrhage and bleed uncontrollably. | |
| Such uncontrolled bleeding from collisions, blows and falls is also the leading cause of death among victims age 5 to 44 in the United States. | |
| Natural blood platelets are the key ingredient to stopping bleeding, a process called hemostasis. The process works well for typical cuts and scrapes, but can be overwhelmed with serious injuries. | |
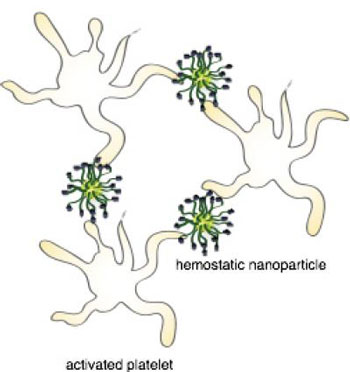 | |
| This is a schematic of hemostatic nanoparticles linking with blood platelets. The nanoparticles significantly increased survival rate from blast trauma in preclinical testing. (Image: Erin Lavik) | |
| Hospitals try to stem internal bleeding by giving trauma patients blood products or the hemophilia medicine called recombinant factor VIIa, but there isn't a good option for the battlefield or accident scenes. Recombinant factor VIIa must be refrigerated, costs up to tens of thousands of dollars per treatment and can cause clots in brain and spinal cord injuries, which are common from explosions. | |
| Lavik's team has fine-tuned the nanoparticles to increase clotting efficiency. "They are incredibly simple… spheres with arms of peptides that react with activated blood platelets in damaged tissues to help clots form more quickly," she said. | |
| The particles are made from short polymer chains already approved for other uses by the U.S. Food and Drug Administration. In earlier testing, rat models injected with the nanoparticles stopped bleeding faster than untreated models. | |
| The dry particles remained viable after two weeks on a shelf. A medic in the field or an ambulance crew would add saline, shake and inject them, the researchers say. | |
| Further research and testing are underway. Clinical trials on humans are likely at least five years out, Lavik said. |
Labels:
EMS,
nano-technology
Tuesday, June 3, 2014
A device to control bleeding in brain surgery receives Phase 2 SBIR grant
 A medical device developer got a big boost in its efforts to develop a surgical sealant for brain surgery. Endomedix received a $1.49 million Phase 2 Small Business Innovation Research grant to help control bleeding for surgical procedures, according to a company statement. It received the grant from the National Institute of Neurological Disorders and Stroke.
A medical device developer got a big boost in its efforts to develop a surgical sealant for brain surgery. Endomedix received a $1.49 million Phase 2 Small Business Innovation Research grant to help control bleeding for surgical procedures, according to a company statement. It received the grant from the National Institute of Neurological Disorders and Stroke.The funding will go toward safety studies, in vivo performance studies and developing an applicator device. The sealant is a hydrogel that includes two processed biocompatible polysaccharides. They are simultaneously mixed and sprayed onto a surgical site.
Typically, a Phase 2 SBIR grant doesn’t exceed $1 million over a two-year period.
The market value for sealants and related devices is expected to top $4.2 billion in 2018. But some factors that could impact the size of the market include the efforts to limit expensive hospital admissions, shift procedures to outpatient settings and reduce rates of re-admissions.
Endomedix has won six SBIR Phase I grants totaling more than $1.1 million to advance its sealants program.
The Newark medical device company is based at the Enterprise Development Center incubator at the New Jersey Institute of Technology.
Labels:
Endomedix
Sunday, May 18, 2014
Cohera Medical, Inc.® Files for CE Mark Approval for Sylys® Surgical Sealant
PITTSBURGH, May 15, 2014 /PRNewswire/ -- Cohera Medical, Inc.®, a leading innovator and developer of absorbable surgical adhesives and sealants, announced today that it has applied for CE Mark approval for Sylys® Surgical Sealant, the first synthetic sealant designed specifically to help reduce anastomotic leaks in gastrointestinal procedures. CE Mark approval, expected by the end of 2014 based on this filing, will allow for marketing of the product in the European Union and other countries.
Sylys is applied during gastrointestinal procedures to help prevent the occurrence of anastomotic leaks – a serious complication that occurs in up to 23 percent of patients undergoing colorectal surgery. At least one-third of the post-surgical mortality after colorectal surgery is attributed to leaks, and survivors generally have protracted recoveries. The additional care required to manage this serious complication can cause up to a five-fold increase in patient management costs.
"Anastomotic leakage is the most devastating complication associated with intestinal resection, contributing to morbidity and mortality," said James McCormick, DO, FACS, FASCRS, Chief, Division of Colorectal Surgery, The Western Pennsylvania Hospital. "We have made tremendous strides in curtailing the risk associated with intestinal anastomosis, but we are always striving for further improvement and greater patient safety."
Sylys is designed to help reduce anastomotic leakage in intestinal procedures by providing additional support during the first few days of healing, when the development of leaks is most likely to occur. The sealant is applied as a viscous fluid that cures rapidly to create a flexible, elastic seal over the anastomosis site.
"The preparation and filing of the CE Mark application for Sylys, a Class III implant technology, represents a significant milestone and achievement by all of the employees of Cohera Medical," said Chad A. Coberly, JD, Vice President of Clinical, Regulatory and Legal Affairs for Cohera Medical. "The submission signifies that we have completed the rigorous clinical and pre-clinical testing, quality, and performance requirements of the EU authorities, and we look forward to working with our notified body during the approval process."
The market for Sylys is significant, with several hundred thousand procedures a year worldwide representing a multi-billion dollar opportunity. Due to the unmet clinical need, Sylys would represent a breakthrough in this market that will lead to improved patient outcomes as well as reduced patient management costs for healthcare providers.
"Submitting the Sylys CE Mark application represents a significant milestone towards the commercialization of our second product and demonstrates the commitment we have to our future customers, partners, and investors," said Patrick Daly, Cohera Medical president and CEO. "We look forward to making Sylys available to surgeons and patients throughout the world."
Labels:
Cohera Medical,
gastrointestinal sealant,
sealant,
sylys
Monday, May 5, 2014
Z-Medica Bolsters Hemostatic Product Line with Acquisition of Novacol®
WALLINGFORD, CONN. — Z-Medica, a leading developer and marketer of hemostatic agents, today announced that they have signed a definitive agreement with TAUREON, headquartered in The Netherlands, to acquire Novacol®, a Class III resorbable hemostatic product.
Novacol is currently sold in Europe and South Korea. Z-Medica will continue to support that marketing strategy while making plans to expand the product offering into other countries.
“Given our QuikClot portfolio of hemostatic dressings, it was a natural fit for us to augment both our product line and our international presence with Novacol,” said Z-Medica’s President and CEO Stephen J. Fanning. “Our current products are gauze-based and non-resorbable. Acquiring Novacol, which is a resorbable hemostat, is the first step towards expanding the Z-Medica portfolio and providing a wider range of innovative, safe, and effective products to the broader healthcare market.”
Used primarily in surgeries, Novacol is comprised of 100% high-quality, purified, natural long and short collagen fiber, which is resorbed by the body. The product is available as the soft, pliable Novacol® Pad, and Novacol® Fibrillar for use in cavities and irregular surfaces.
“Novacol is an effective product that will benefit from Z-Medica’s global distribution network,” said TAUREON CEO Dick van Kalkeren. “Z-Medica’s experience with QuikClot and their reputation as a leader in the hemostatic market ensures that Novacol will be able to compete favorably in the market. The transfer of Novacol also enables TAUREON to strengthen our focus in Europe on plastic and orthopedic surgery, human tissue and advanced wound care.”
Novacol is currently sold in Europe and South Korea. Z-Medica will continue to support that marketing strategy while making plans to expand the product offering into other countries.
“Given our QuikClot portfolio of hemostatic dressings, it was a natural fit for us to augment both our product line and our international presence with Novacol,” said Z-Medica’s President and CEO Stephen J. Fanning. “Our current products are gauze-based and non-resorbable. Acquiring Novacol, which is a resorbable hemostat, is the first step towards expanding the Z-Medica portfolio and providing a wider range of innovative, safe, and effective products to the broader healthcare market.”
Used primarily in surgeries, Novacol is comprised of 100% high-quality, purified, natural long and short collagen fiber, which is resorbed by the body. The product is available as the soft, pliable Novacol® Pad, and Novacol® Fibrillar for use in cavities and irregular surfaces.
“Novacol is an effective product that will benefit from Z-Medica’s global distribution network,” said TAUREON CEO Dick van Kalkeren. “Z-Medica’s experience with QuikClot and their reputation as a leader in the hemostatic market ensures that Novacol will be able to compete favorably in the market. The transfer of Novacol also enables TAUREON to strengthen our focus in Europe on plastic and orthopedic surgery, human tissue and advanced wound care.”
Wednesday, April 23, 2014
The Medicines Company Adds Novel, Approved, Surgical Sealant to Its Surgical Hemostasis Portfolio Acquires Tenaxis Medical, Inc.
PARSIPPANY, NJ and MOUNTAIN VIEW, CA -- (Marketwired) -- 04/23/14 -- The Medicines Company (NASDAQ: MDCO) and Tenaxis Medical, Inc. (Tenaxis) today announced an agreement for The Medicines Company to acquire Tenaxis. Tenaxis's sole product, which mechanically seals both human tissue and artificial grafts is approved, but not launched in the US -- having received US PMA approval from the FDA in March 2013 as a vascular sealant. The product is also approved with a European CE Mark as a surgical sealant applicable to cardiovascular, general, urological, and thoracic surgery. The addition of the Tenaxis product adds another solution for surgical bleeding to The Medicines Company's portfolio which also includes the marketed product, RecoThrom (aqueous, recombinant human Thrombin) and the investigational product, Fibrocaps (a dry powder formulation of fibrinogen and thrombin being developed to aid in hemostasis during surgery) which has completed phase III trials and is under FDA and EMA review.
Under the terms of the agreement, The Medicines Company will pay $58 million upfront on closing of the deal. The Medicines Company will also pay milestone payments of up to $112 million contingent upon achieving certain commercial and -- in pursuit of even broader indications -- regulatory approval milestones. The transaction is subject to the satisfaction of customary closing conditions.
"We continue to execute our strategy for growth, building our presence in surgery and perioperative care," said Clive Meanwell, Chairman and Chief Executive Officer of The Medicines Company.
Adam Sharkawy, Senior Vice President and Global Innovation Group Leader for Surgery and Perioperative Care added, "A robust portfolio of solutions for intra-operative bleeding is expected to drive growth for us in this sector of hospital medicine. This acquisition will allow us to leverage and build our activities in surgery centers at leading US and European hospitals. In the US, we expect to deploy approximately 100 of our current engagement managers across these surgical product offerings."
In a pivotal trial in vascular surgery, the Tenaxis sealant was effective when used as prophylactic treatment on native vessels and grafts, reducing the incidence of bleeding within the first minute after removal of vascular clamps. The Tenaxis sealant was compared to Gelfoam Plus, a topical hemostat containing a low concentration of thrombin (125 Units/mL), in the clinical trial used to support licensure (N=217; 1:1 randomization). The Tenaxis surgical sealant was shown to be superior to Gelfoam Plus based on a statistically significantly lower incidence of suture hole bleeding at the time of clamp release (60.5% vs. 39.6% of anastomotic sites at Time 0; p = 0.0001); the 20% difference at the time of clamp release persisted at 10 minutes (82% vs. 72%). Superiority was demonstrated in several types of surgical procedures (extremity bypass, hemodialysis access grafting, and other vascular procedures).
"We are excited to be involved in a transaction with The Medicines Company, which will allow more patients to have access to this beneficial technology," Ronald Dieck, President and CEO of Tenaxis commented. "We are proud of the surgical sealant technologies that we have developed and their impact on the wellbeing of patients. The Medicines Company is clearly committed to the area of intraoperative hemostasis and we look forward to working as a team to innovate in this area of medicine."
The Boards of Directors of both companies have unanimously approved the agreement.
Gibbons P.C. served as legal advisor for the transaction for The Medicines Company. Leerink Swann & Co. served as financial advisor and Wilson Sonsini Goodrich & Rosati, PC served as legal advisor for the transaction for Tenaxis Medical, Inc.
Conference Call Information
There will be a conference call with The Medicines Company management today at 8:30 a.m. Eastern Time to discuss the Tenaxis acquisition, first quarter 2014 financial results, operational developments, and outlook. The conference call will be available via phone and webcast. The webcast can be accessed at www.themedicinescompany.com.
Domestic Dial In: +1 (877) 359-9508
International Dial In: +1 (224) 357-2393
Passcode for both dial in numbers: 27882505
Replay is available from 11:30 a.m. Eastern Time following the conference call through May 7, 2014. To hear a replay of the call dial +1 855 859-2056 (domestic) and +1 404 537-3406 (international). Passcode for both dial in numbers is 27882505.
Under the terms of the agreement, The Medicines Company will pay $58 million upfront on closing of the deal. The Medicines Company will also pay milestone payments of up to $112 million contingent upon achieving certain commercial and -- in pursuit of even broader indications -- regulatory approval milestones. The transaction is subject to the satisfaction of customary closing conditions.
"We continue to execute our strategy for growth, building our presence in surgery and perioperative care," said Clive Meanwell, Chairman and Chief Executive Officer of The Medicines Company.
Adam Sharkawy, Senior Vice President and Global Innovation Group Leader for Surgery and Perioperative Care added, "A robust portfolio of solutions for intra-operative bleeding is expected to drive growth for us in this sector of hospital medicine. This acquisition will allow us to leverage and build our activities in surgery centers at leading US and European hospitals. In the US, we expect to deploy approximately 100 of our current engagement managers across these surgical product offerings."
In a pivotal trial in vascular surgery, the Tenaxis sealant was effective when used as prophylactic treatment on native vessels and grafts, reducing the incidence of bleeding within the first minute after removal of vascular clamps. The Tenaxis sealant was compared to Gelfoam Plus, a topical hemostat containing a low concentration of thrombin (125 Units/mL), in the clinical trial used to support licensure (N=217; 1:1 randomization). The Tenaxis surgical sealant was shown to be superior to Gelfoam Plus based on a statistically significantly lower incidence of suture hole bleeding at the time of clamp release (60.5% vs. 39.6% of anastomotic sites at Time 0; p = 0.0001); the 20% difference at the time of clamp release persisted at 10 minutes (82% vs. 72%). Superiority was demonstrated in several types of surgical procedures (extremity bypass, hemodialysis access grafting, and other vascular procedures).
"We are excited to be involved in a transaction with The Medicines Company, which will allow more patients to have access to this beneficial technology," Ronald Dieck, President and CEO of Tenaxis commented. "We are proud of the surgical sealant technologies that we have developed and their impact on the wellbeing of patients. The Medicines Company is clearly committed to the area of intraoperative hemostasis and we look forward to working as a team to innovate in this area of medicine."
The Boards of Directors of both companies have unanimously approved the agreement.
Gibbons P.C. served as legal advisor for the transaction for The Medicines Company. Leerink Swann & Co. served as financial advisor and Wilson Sonsini Goodrich & Rosati, PC served as legal advisor for the transaction for Tenaxis Medical, Inc.
Conference Call Information
There will be a conference call with The Medicines Company management today at 8:30 a.m. Eastern Time to discuss the Tenaxis acquisition, first quarter 2014 financial results, operational developments, and outlook. The conference call will be available via phone and webcast. The webcast can be accessed at www.themedicinescompany.com.
Domestic Dial In: +1 (877) 359-9508
International Dial In: +1 (224) 357-2393
Passcode for both dial in numbers: 27882505
Replay is available from 11:30 a.m. Eastern Time following the conference call through May 7, 2014. To hear a replay of the call dial +1 855 859-2056 (domestic) and +1 404 537-3406 (international). Passcode for both dial in numbers is 27882505.
Labels:
Medicines Company,
sealant,
Tenaxis
Saturday, April 5, 2014
FDA Accepts Biologic License Application for Fibrocaps Hemostatic Agent
The Medicines Company has announced that the U.S. Food and Drug Administration (FDA) has accepted the filing of a biologic license application (BLA) for the investigational hemostatic agent Fibrocaps, a dry powder formulation of fibrinogen and thrombin being developed to aid in hemostasis during surgery, where control of mild or moderate bleeding by conventional means is ineffective or impractical. The FDA action date (PDUFA date) for Fibrocaps is January 31, 2015.
In August 2013, The Medicines Company announced that the Phase III trial, FINISH-3, which studied a total of 719 patients across 54 sites in the U.S. and Western Europe, met all of its primary and secondary hemostasis efficacy endpoints in each of four distinct populations: (1) spinal surgery; (2) hepatic resection; (3) soft tissue dissection; and (4) vascular surgery.
"We believe Fibrocaps has the potential to become an important hemostatic product -- complementary to Recothrom® Thrombin, topical (Recombinant) -- which will allow us to continue to serve leading US hospitals, leveraging our existing operations. Our previously announced EMA filing also suggests that Fibrocaps can be our first hemostat product in the European market," said Adam Sharkawy, PhD, Senior Vice President, and Surgery and Perioperative Care Global Innovation Group Leader at The Medicines Company.
"With the acceptance of the Fibrocaps BLA we now have 6 new molecular entity regulatory submissions under review at the FDA and the EMA," said Clive Meanwell, MD, PhD, Chairman and Chief Executive Officer of The Medicines Company. "These applications span our three areas of focus in leading hospitals, namely: acute cardiovascular care, surgery and perioperative care, and serious infectious disease care. Each is designed to contribute to our purpose which is to save lives, alleviate suffering, and contribute to the economics of healthcare by focusing on the needs of leading hospitals worldwide."
In August 2013, The Medicines Company announced that the Phase III trial, FINISH-3, which studied a total of 719 patients across 54 sites in the U.S. and Western Europe, met all of its primary and secondary hemostasis efficacy endpoints in each of four distinct populations: (1) spinal surgery; (2) hepatic resection; (3) soft tissue dissection; and (4) vascular surgery.
"We believe Fibrocaps has the potential to become an important hemostatic product -- complementary to Recothrom® Thrombin, topical (Recombinant) -- which will allow us to continue to serve leading US hospitals, leveraging our existing operations. Our previously announced EMA filing also suggests that Fibrocaps can be our first hemostat product in the European market," said Adam Sharkawy, PhD, Senior Vice President, and Surgery and Perioperative Care Global Innovation Group Leader at The Medicines Company.
"With the acceptance of the Fibrocaps BLA we now have 6 new molecular entity regulatory submissions under review at the FDA and the EMA," said Clive Meanwell, MD, PhD, Chairman and Chief Executive Officer of The Medicines Company. "These applications span our three areas of focus in leading hospitals, namely: acute cardiovascular care, surgery and perioperative care, and serious infectious disease care. Each is designed to contribute to our purpose which is to save lives, alleviate suffering, and contribute to the economics of healthcare by focusing on the needs of leading hospitals worldwide."
Labels:
fibrocaps,
recombinant,
recothrom
Sunday, March 23, 2014
Drug companies developing longer-acting clotting agents for hemophiliacs
Several drug companies, such as Biogen Idec and Novo Nordisk, are developing new, longer-acting versions of the blood clotting factors used by people with hemophilia. Patients with severe forms of the disease need regular infusions, lasting 30 minutes or more, of relatively short acting and very expensive clotting factors.
The new longer-lasting hemophilia B products can be given every 10 days or two weeks, offering significant advantages for patients, especially young children, who now need infusions every two or three days.
Hemophilia is hereditary, passed from parent to child through genes. People with hemophilia have little or no clotting factor. Hemophilia A and Hemophilia B have different clotting factors that are low or missing, but both can experience spontaneous bleeding, as well as severe bleeding following injuries or surgery.
Worldwide, about one in 5,000 men is born with hemophilia A and one in 25,000 men is born with hemophilia B each year. Since the gene is carried on the X chromosome, hemophilia is almost entirely a disease of men. Women can pass the gene to their offspring. Hemophilia has often been called the “Royal Disease” since it was carried by Britain’s Queen Victoria and affected many of the ruling families of Europe. Blood factor concentrates were not developed until the mid-20th century, and up until that time people with hemophilia had a life expectancy of less than 30 years.
The U.S. Food and Drug Administration is due to decide by mid-year whether to approve a new long-lasting hemophilia B clotting factor from Biogen Idec. Novo Nordisk expects to file next year for regulatory approval of its long-acting hemophilia B drug.
Some industry experts say these and other new treatments could help drive down the price of existing hemophilia products, which can total $300,000 or more a year for a single patient.
The new longer-lasting hemophilia B products can be given every 10 days or two weeks, offering significant advantages for patients, especially young children, who now need infusions every two or three days.
Hemophilia is hereditary, passed from parent to child through genes. People with hemophilia have little or no clotting factor. Hemophilia A and Hemophilia B have different clotting factors that are low or missing, but both can experience spontaneous bleeding, as well as severe bleeding following injuries or surgery.
Worldwide, about one in 5,000 men is born with hemophilia A and one in 25,000 men is born with hemophilia B each year. Since the gene is carried on the X chromosome, hemophilia is almost entirely a disease of men. Women can pass the gene to their offspring. Hemophilia has often been called the “Royal Disease” since it was carried by Britain’s Queen Victoria and affected many of the ruling families of Europe. Blood factor concentrates were not developed until the mid-20th century, and up until that time people with hemophilia had a life expectancy of less than 30 years.
The U.S. Food and Drug Administration is due to decide by mid-year whether to approve a new long-lasting hemophilia B clotting factor from Biogen Idec. Novo Nordisk expects to file next year for regulatory approval of its long-acting hemophilia B drug.
Some industry experts say these and other new treatments could help drive down the price of existing hemophilia products, which can total $300,000 or more a year for a single patient.
Labels:
novo nordisk
Saturday, March 22, 2014
Mercy adopts new blood cell transfusion guidelines
SIOUX CITY | Mercy Medical Center -- Sioux City has stepped up efforts to conserve red blood cells.
All hospitals in the Trinity Health network have applied the new national recommendations developed by The Joint Commission and the American Medical Association -- convened Physician Consortium of Performance Improvement that advise adopting a "more restrictive" practice of red blood cell transfusion to produce better patient outcomes.
"We want to give just what is necessary, one unit at a time," said Dr. Gregg Galloway, a pathologist and vice chair of the Infectious Disease Committee at Mercy. "Give him a unit of blood. It will make him feel better -- that's the old paradigm. We don't believe it anymore."
Mercy has always had guidelines in place instructing staff when to give red blood cell transfusions, but Galloway said those rules have significantly changed and are "more restrictive" than they used to be.
"We're doing it because we do believe it is better quality patient care at all health care institutions and because of the cost of the resources," he said.
The new guidelines were released following a September 2012 national summit that convened representatives from 112 professional organizations and associations in effort to curb overuse of five medical treatments. Transfusion of red blood cells in hospitals was one of them.
A decade of studies, according to Galloway, found that when "more restrictive" red blood cell transfusion practices were used, hospitalized patients had lower mortality rates, fewer complications from infectious diseases, less breathing problems and cardiac events, as well as a reduced chance of developing acute respiratory distress syndrome -- a life-threatening lung condition that prevents enough oxygen from getting to the lungs and into the blood.
Galloway said hospital staff give patients red blood cell transfusions based on their hemoglobin (a protein in red blood cells that carries oxygen) and hermatocrit values (percentage of red blood cells found in whole blood). When their hemoglobin value dropped below 10 grams per deciliter (G/dL), the patients received a transfusion. The "more restrictive" guidelines, he said, don't recommend transfusion until hemoglobin values fall between 7 and 8 G/dL.
"Big difference. You're going to use less red cell and units in the hospital," he said. "We predict that overall we'll have a 15 to 20 percent drop over the next year and a half to two years in our red cells that we'll use in our hospital setting."
Once blood is collected at a donation center, it's processed, placed in a refrigerator and stored. Chemical changes, he said, occur in the hemoglobin, and red blood cells lose their malleability.
"Those red blood cells start to change," he said. "They are no longer like the normal red blood cells you and I have floating around in our body. Banked blood is a tissue transplant. Those are somebody else's red cells."
In addition to the new guidelines, Galloway said, improvements in surgical techniques that reduce the need for red blood cell transfusions in the operating room are also contributing to the downward trend.
Surgeons are encouraged to salvage patients' blood in the operating room, a practice that was once reserved for open heart surgery. Now it's being used in trauma, abdominal aneurism and orthopedic surgeries.
"We're trying to save the patient's own blood. We re-process it in the OR and we give it back to them," Galloway said. "We also have new hemostatic medications that we can give patients that keep them from bleeding as much in OR."
Large hospitals, he said, now have blood management programs that staff nurses who review a patient's clinical situation alongside the guidelines. Some patients seeking elective surgery, he said, may have to wait longer to get into the operating room if they lack enough healthy red blood cells.
Labels:
infection,
transfusion
Adhesion Incidence and Severity Vary by Surgical Procedure
Experts estimate approximately 93% of patients who have undergone laparoscopic surgery develop abnormal fibroid bands that bind organ surfaces to the abdominal wall after a second surgery. Many adhesions are asymptomatic, but in some patients, they can cause pain, small bowel obstruction and other postoperative issues, as well as increase cost and complicate surgical suite workload. As surgeons underestimate the rate of adhesion development given that up to 93% of consent forms don’t address them, the March 2014 issue of Surgery Today contained a systematic review estimating the formation rate, distribution, and severity of postoperative adhesions in abdominal surgery patients.
After reviewing 25 studies published between January 1990 and July 2011, the authors determined the weighted mean formation rate of adhesions after abdominal surgery was 54%. However, different types of surgeries and procedures had different adhesion statistics. Among patients who had gastrointestinal (GI) surgery, 66% developed adhesions. In patients who had gynecologic and urologic surgeries, the rates were 51% and 22%, respectively.
Since those rates were lower than the numbers reported a few decades ago, the authors suggested current practices such as minimizing peritoneal foreign body exposure, careful tissue handling, meticulous hemostasis, specific closure of the peritoneum in caesarean sections, and modern surgical techniques have helped reduce the rate of adhesion.
The authors reported laparoscopic surgery reduced the adhesion formation rate by 25% and also decreased the adhesion severity score for Gl surgery. They concluded heightening awareness of adhesions is needed to shed light on the complication’s etiology. With better understanding of how and why adhesions form, researchers will be able to develop novel therapeutic approaches, the authors said. They also suggested the potential for adhesions should be addressed clearly in consent forms.
After reviewing 25 studies published between January 1990 and July 2011, the authors determined the weighted mean formation rate of adhesions after abdominal surgery was 54%. However, different types of surgeries and procedures had different adhesion statistics. Among patients who had gastrointestinal (GI) surgery, 66% developed adhesions. In patients who had gynecologic and urologic surgeries, the rates were 51% and 22%, respectively.
Since those rates were lower than the numbers reported a few decades ago, the authors suggested current practices such as minimizing peritoneal foreign body exposure, careful tissue handling, meticulous hemostasis, specific closure of the peritoneum in caesarean sections, and modern surgical techniques have helped reduce the rate of adhesion.
The authors reported laparoscopic surgery reduced the adhesion formation rate by 25% and also decreased the adhesion severity score for Gl surgery. They concluded heightening awareness of adhesions is needed to shed light on the complication’s etiology. With better understanding of how and why adhesions form, researchers will be able to develop novel therapeutic approaches, the authors said. They also suggested the potential for adhesions should be addressed clearly in consent forms.
Labels:
adhesion,
BioCer,
laparoscopic
Friday, February 7, 2014
Popular Blood-thinner Manufacturer Didn’t Want Internal Research Made Public
Boehringer Ingelheim, the manufacturer of the blood-thinning drug Pradaxa, was concerned that releasing the results of an internal research paper on the drug would damage drug sales, records recently made public show. The company was so worried about the results of the study that some employees pressured the author to revise it, and the company recommended it be thrown out, according to a recent report by the New York Times.
Records recently made public by a federal judge in Illinois presiding over thousands of lawsuits against the maker of Pradaxa, including emails, internal memos, and presentations, centered on the research project and whether it would be damaging to Pradaxa’s main selling point: that users of the drug aren’t required to undergo regular blood work while taking Pradaxa.
Pradaxa (dabigatran) was approved in 2010 as an alternative to an existing anti-clotting drug, warfarin. Both are anticoagulants used to prevent and treat blood clots and reduce the risk of stroke, but Boehringer Ingelheim marketed its drug as less of a nuisance than warfarin, which requires frequent blood tests and careful monitoring.
Unfortunately, Pradaxa has been linked to more than 1,000 deaths in the United States. Since 2010, an unprecedented number of adverse events related to the drug have been reported to the FDA. Experts have also questioned the reliability of an FDA study affirming the safety of Pradaxa.
Pradaxa has claimed superiority to warfarin. However, documents prepared by the FDA clearly state that “It is important… not to provide dabigatran with a superiority claim to warfarin, because it would imply that even those well-treated with warfarin should be switched to dabigatran. Clearly, that is not the case.”
Furthermore, a research paper written by Paul. A. Reilly, a clinical program director with Boehringer Ingelheim indicates that patients could benefit from having their blood monitored while taking Pradaxa. Reilly states that some patients absorb too little of the drug, rendering it ineffective, while some absorb so much that their risk for bleeding increases.
According to the New York Times, after Reilly’s paper was circulated within the company, Dr. Jutta Heinrich-Nols, a company supervisor, sent an email stating that she couldn’t believe the company was planning to publish Reilly’s work. She warned that publishing his results could make it “extremely difficult” for the company to maintain its claims that patients taking Pradaxa do not need regular blood tests.
Heinrich-Nols also noted that Reilly’s research could “undermine” the company’s efforts to compete with other new anticoagulants like Xarelto and Eliquis. The marketing of Pradaxa is yet another example of the dangers that consumers face when safety information is regulated by pharmaceutical companies primarily concerned with profit.
Boehringer Ingelheim issued a statement saying that the recently-released documents “represent small fragments of the robust discussion and debate that is a vital component in all scientific inquiry and in the research and development of any important medication such as Pradaxa.”
Unlike warfarin, Pradaxa has no antidote to reverse its blood-thinning effects. Despite issues with the FDA’s approach to approving the drug, as well as numerous patient bleeding deaths and adverse event reports, Pradaxa remains on the market as a safe drug.
Records recently made public by a federal judge in Illinois presiding over thousands of lawsuits against the maker of Pradaxa, including emails, internal memos, and presentations, centered on the research project and whether it would be damaging to Pradaxa’s main selling point: that users of the drug aren’t required to undergo regular blood work while taking Pradaxa.
Pradaxa (dabigatran) was approved in 2010 as an alternative to an existing anti-clotting drug, warfarin. Both are anticoagulants used to prevent and treat blood clots and reduce the risk of stroke, but Boehringer Ingelheim marketed its drug as less of a nuisance than warfarin, which requires frequent blood tests and careful monitoring.
Unfortunately, Pradaxa has been linked to more than 1,000 deaths in the United States. Since 2010, an unprecedented number of adverse events related to the drug have been reported to the FDA. Experts have also questioned the reliability of an FDA study affirming the safety of Pradaxa.
Pradaxa has claimed superiority to warfarin. However, documents prepared by the FDA clearly state that “It is important… not to provide dabigatran with a superiority claim to warfarin, because it would imply that even those well-treated with warfarin should be switched to dabigatran. Clearly, that is not the case.”
Furthermore, a research paper written by Paul. A. Reilly, a clinical program director with Boehringer Ingelheim indicates that patients could benefit from having their blood monitored while taking Pradaxa. Reilly states that some patients absorb too little of the drug, rendering it ineffective, while some absorb so much that their risk for bleeding increases.
According to the New York Times, after Reilly’s paper was circulated within the company, Dr. Jutta Heinrich-Nols, a company supervisor, sent an email stating that she couldn’t believe the company was planning to publish Reilly’s work. She warned that publishing his results could make it “extremely difficult” for the company to maintain its claims that patients taking Pradaxa do not need regular blood tests.
Heinrich-Nols also noted that Reilly’s research could “undermine” the company’s efforts to compete with other new anticoagulants like Xarelto and Eliquis. The marketing of Pradaxa is yet another example of the dangers that consumers face when safety information is regulated by pharmaceutical companies primarily concerned with profit.
Boehringer Ingelheim issued a statement saying that the recently-released documents “represent small fragments of the robust discussion and debate that is a vital component in all scientific inquiry and in the research and development of any important medication such as Pradaxa.”
Unlike warfarin, Pradaxa has no antidote to reverse its blood-thinning effects. Despite issues with the FDA’s approach to approving the drug, as well as numerous patient bleeding deaths and adverse event reports, Pradaxa remains on the market as a safe drug.
Labels:
anti-coagulants,
Pradaxa
Arch Therapeutics offers $2.9M in private stock placement
Massachusetts-based Arch Therapeutics hopes to sell over 11 million shares to raise close to $3 million in support of its medical sealants and hemostasis products.
Arch Therapeutics brings in nearly $3M in private stock placement
Arch Therapeutics launched a private placement fundraising effort, offering 11.4 million shares of common stock in hopes of raising $2.9 million.
Wellesley, Mass.-based Arch Therapeutics develops medical sealants and hemostasis products for use during surgeries. The company's marquee device is the AC5 Surgical Hemostatic for minimally invasive and open surgical procedures.
Arch is offering unnamed "institutional and high net worth" investors 11.4 million shares of common stock at a price of 25¢ per share. Investors will also receive warrants to purchase up to 11.4 million additional shares at an exercise price of either 30¢, 35¢ or 40¢ apiece, depending on whether investors purchase from the Series A, B or C warrants, according to a press release.
Arch Therapeutics completed a share swap and reverse merger, to expand into a life sciences company. Arch reported a $7.3 million financing through a share-swap related to its reverse merger with Arch Biosurgery, which expanded the company's range of products and services.
Arch Therapeutics brings in nearly $3M in private stock placement
Arch Therapeutics launched a private placement fundraising effort, offering 11.4 million shares of common stock in hopes of raising $2.9 million.
Wellesley, Mass.-based Arch Therapeutics develops medical sealants and hemostasis products for use during surgeries. The company's marquee device is the AC5 Surgical Hemostatic for minimally invasive and open surgical procedures.
Arch is offering unnamed "institutional and high net worth" investors 11.4 million shares of common stock at a price of 25¢ per share. Investors will also receive warrants to purchase up to 11.4 million additional shares at an exercise price of either 30¢, 35¢ or 40¢ apiece, depending on whether investors purchase from the Series A, B or C warrants, according to a press release.
Arch Therapeutics completed a share swap and reverse merger, to expand into a life sciences company. Arch reported a $7.3 million financing through a share-swap related to its reverse merger with Arch Biosurgery, which expanded the company's range of products and services.
Labels:
Arch Therapeutics
Tuesday, January 28, 2014
Baxter sues Johnson & Johnson over FloSeal patents
Baxter (NYSE:BAX) accused Johnson & Johnson (NYSE:JNJ) of infringing 6 patents covering its FloSeal line with the Ethicon SurgiFlo line of competing surgical hemostasis products.
In a lawsuit filed last week in the U.S. District Court for Northern Illinois, Baxter said the alleged infringement is willful and asked Judge Sharon Johnson Coleman for triple damages, pre- and post-judgment interest, legal costs and a jury trial.
"Defendants' SurgiFlo products directly compete with Baxter's biosurgery products, including the Floseal family of products, which practice the Patents-in-Suit. On information and belief, defendants are aware of Floseal's established position in the hemostatic products market and carefully track Baxter's marketing and other activities related to the Floseal products in the United States and worldwide. For example, one or more of Defendants have repeatedly communicated with Baxter regarding competitive marketing issues in the United States and abroad pertaining to Floseal and SurgiFlo," Baxter alleged, according to court documents. "Defendants have infringed and will continue to infringe Baxter's intellectual property rights by making, using, selling, offering for sale within the United States and/or importing into the United States delivery products for hemostasis such as the SurgiFlo family of products."
In a lawsuit filed last week in the U.S. District Court for Northern Illinois, Baxter said the alleged infringement is willful and asked Judge Sharon Johnson Coleman for triple damages, pre- and post-judgment interest, legal costs and a jury trial.
"Defendants' SurgiFlo products directly compete with Baxter's biosurgery products, including the Floseal family of products, which practice the Patents-in-Suit. On information and belief, defendants are aware of Floseal's established position in the hemostatic products market and carefully track Baxter's marketing and other activities related to the Floseal products in the United States and worldwide. For example, one or more of Defendants have repeatedly communicated with Baxter regarding competitive marketing issues in the United States and abroad pertaining to Floseal and SurgiFlo," Baxter alleged, according to court documents. "Defendants have infringed and will continue to infringe Baxter's intellectual property rights by making, using, selling, offering for sale within the United States and/or importing into the United States delivery products for hemostasis such as the SurgiFlo family of products."
Thursday, January 9, 2014
Sealant Inspired By Beach Worm Could Become Surgical Superglue
Remember that wacky glue commercial from the 1980s? "Krazy Glue, you crazy rat," the narrator says. "Strong enough to hold this man suspended in mid-air." He promises the stuff can bond almost anything: a plastic knob, a plastic plug, a rubber boot, a door knob, and even a flashlight case.
Heck, a version of the everlasting adhesive is even approved by the Food and Drug Administration to seal skin wounds.
But superglue can't fix a broken heart — or even a torn artery. Yet.
Now a team of doctors and engineers at Brigham and Women's Hospital in Boston are getting close to changing that. Their unlikely inspiration is a 3-inch worm that lives off the coast of California.
Cardiac surgeon Pedro del Nido and his colleagues have developed a biodegradable adhesive that can patch a hole in a pig's heart or artery. The experimental glue is nontoxic and is strong enough to hold up under the high pressures in the human heart, the team report Wednesday in the journal Science Translational Medicine.
So far, they've tested the glue only in animals. So the sealant is far from reaching the operating room or battlefield. But del Nido hopes the adhesive will eventually replace traditional sutures and staples for some operations, especially heart surgery.
"A glue is the holy grail for repairing hearts," del Nido tells Shots. "Right now we use sutures. Every time the needle and thread enter normal tissue, they do a little bit of damage. Usually it doesn't matter. But I repair children's hearts. For those, this damage can really be a problem."
Regular superglues don't work well inside the body. "It's a skin glue," del Nido says. "You can't use it internally because it hardens as soon as it comes into contact with water." And the glues are made from a compound called cyanoacrylate, which can be toxic.
To find a safe adhesive that could work on hearts, arteries and other organ surfaces, del Nido teamed up with bioengineer Jeffrey Karp, also at Brigham and Women's Hospital.
"In our lab, we look to nature for inspiration in designing materials," Karp tells Shots. "Solutions are really all around us." Karp's lab has been looking at porcupine quills for insights that could lead to better surgical needles.
The barbs on porcupine quills make it easier from them to penetrate the skin.
For the heart glue, Karp and his team turned their attention to critters that stick to slippery surfaces, such as slugs, spiders and a bristly little worm that glues itself rocks in tidal pools, called thesandcastle worm.
To eat, chitons use their teeth, which look like black bulbs with bluish highlights, to grind up rock.
"We started looking at how creatures, like the sandcastle worm, could attach to wet surfaces," Karp says. After years of experimenting with various chemical cocktails, he and his team finally stumbled upon an adhesive that's biodegradable and nontoxic.
"Cells and tissues can grow over the material and into it," he says. Eventually it just dissolves into the body. And the glue only hardens when UV light shines on it. So a surgeon can put the glue in exactly the right place before it seals up.
Although Karp and del Nido haven't tested the experimental glue on people yet, they've put the adhesive through a whole battery of tests in animals.
"We made a hole in the heart of a living rat and showed that we can seal it up without removing the blood," Karp says. "The animals were fine six months later."
They also patched a pig's heart and carotid artery with the glue. Even after the pig was given a shot of adrenaline and its heart pressure shot through the roof, the patched stayed on the tissue.
Of course, humans are more complicated than pigs and rats. And the glue has to be safe for decades in people, not months.
But Karp is so confident that the adhesive will one day reach surgeon's toolbox that he has procured $11 million to start a company to manufacture and test the glue. "It appears that the glue is safe," he says. "But we do need to do more studies. We'll repeat the animal tests and then move forward in humans."
Heck, a version of the everlasting adhesive is even approved by the Food and Drug Administration to seal skin wounds.
But superglue can't fix a broken heart — or even a torn artery. Yet.
Now a team of doctors and engineers at Brigham and Women's Hospital in Boston are getting close to changing that. Their unlikely inspiration is a 3-inch worm that lives off the coast of California.
Cardiac surgeon Pedro del Nido and his colleagues have developed a biodegradable adhesive that can patch a hole in a pig's heart or artery. The experimental glue is nontoxic and is strong enough to hold up under the high pressures in the human heart, the team report Wednesday in the journal Science Translational Medicine.
So far, they've tested the glue only in animals. So the sealant is far from reaching the operating room or battlefield. But del Nido hopes the adhesive will eventually replace traditional sutures and staples for some operations, especially heart surgery.
"A glue is the holy grail for repairing hearts," del Nido tells Shots. "Right now we use sutures. Every time the needle and thread enter normal tissue, they do a little bit of damage. Usually it doesn't matter. But I repair children's hearts. For those, this damage can really be a problem."
Regular superglues don't work well inside the body. "It's a skin glue," del Nido says. "You can't use it internally because it hardens as soon as it comes into contact with water." And the glues are made from a compound called cyanoacrylate, which can be toxic.
To find a safe adhesive that could work on hearts, arteries and other organ surfaces, del Nido teamed up with bioengineer Jeffrey Karp, also at Brigham and Women's Hospital.
"In our lab, we look to nature for inspiration in designing materials," Karp tells Shots. "Solutions are really all around us." Karp's lab has been looking at porcupine quills for insights that could lead to better surgical needles.
The barbs on porcupine quills make it easier from them to penetrate the skin.
For the heart glue, Karp and his team turned their attention to critters that stick to slippery surfaces, such as slugs, spiders and a bristly little worm that glues itself rocks in tidal pools, called thesandcastle worm.
To eat, chitons use their teeth, which look like black bulbs with bluish highlights, to grind up rock.
"We started looking at how creatures, like the sandcastle worm, could attach to wet surfaces," Karp says. After years of experimenting with various chemical cocktails, he and his team finally stumbled upon an adhesive that's biodegradable and nontoxic.
"Cells and tissues can grow over the material and into it," he says. Eventually it just dissolves into the body. And the glue only hardens when UV light shines on it. So a surgeon can put the glue in exactly the right place before it seals up.
Although Karp and del Nido haven't tested the experimental glue on people yet, they've put the adhesive through a whole battery of tests in animals.
"We made a hole in the heart of a living rat and showed that we can seal it up without removing the blood," Karp says. "The animals were fine six months later."
They also patched a pig's heart and carotid artery with the glue. Even after the pig was given a shot of adrenaline and its heart pressure shot through the roof, the patched stayed on the tissue.
Of course, humans are more complicated than pigs and rats. And the glue has to be safe for decades in people, not months.
But Karp is so confident that the adhesive will one day reach surgeon's toolbox that he has procured $11 million to start a company to manufacture and test the glue. "It appears that the glue is safe," he says. "But we do need to do more studies. We'll repeat the animal tests and then move forward in humans."
Labels:
super glues
Arch Therapeutics to Webcast Live From the Biotech Showcase Investor Conference
WELLESLEY, MA--(Marketwired - Jan 8, 2014) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), a life sciences company and developer of AC5(TM), a novel product aimed at controlling bleeding and fluid loss in order to provide faster and safer surgical and interventional care, is pleased to announce that Terrence W. Norchi, M.D., CEO of Arch Therapeutics will present at The Biotech Showcase(TM) 2014 conference on Monday, January 13th, at 5:00PM PST. Attendees will find the presentation scheduled for "Track C" in the Mission II room at that time. Dr. Norchi's presentation will provide insights into the Company's ongoing activities to commercially develop novel technologies into a suite of new products targeting markets of unmet clinical need.
A live webcast of Dr. Norchi's Biotech Showcase presentation can be accessed at the following URL: http://www.media-server.com/m/p/8hcwd8zu.
The Biotech Showcase(TM) 2014 Conference (www.ebdgroup.com/bts) takes place January 13-15, 2014 at the Parc 55 Wyndham Hotel, located at Union Square, San Francisco, California. The Biotech Showcase(TM) is an investor and partnering conference devoted to providing private and public biotechnology and life sciences companies an opportunity to present to, and meet with, investors and pharmaceutical executives during the course of one of the industry's largest annual healthcare investor conferences. Now in its sixth year, Biotech Showcase is expected to attract upwards of 1,500 attendees.
About Arch Therapeutics, Inc. (OTCQB: ARTH)
Arch Therapeutics, Inc. is a medical device company developing a novel approach to stop bleeding (hemostasis) and control leaking (sealant) during surgery and trauma care. Arch is developing products based on an innovative self-assembling peptide technology platform to make surgery and interventional care faster and safer for patients. Arch's flagship development stage product candidate, known as AC5(TM), is being designed to achieve hemostasis in minimally invasive and open surgical procedures. Find out more at www.archtherapeutics.com.
Labels:
Arch Therapeutics
Tuesday, January 7, 2014
Z-Medica Signs HealthTrust Agreement
WALLINGFORD, Conn., Jan. 6, 2014 -- /PRNewswire/ -- Z-Medica, a leading developer and marketer of hemostatic agents, announced it has signed an agreement with HealthTrust, a group purchasing and total cost management solutions company, to provide its healthcare member facilities access to QuikClot® products under a vascular closure patch category, effective January 1, 2014.
"Controlling bleeding in a hospital setting is essential for minimizing the length of procedures, reducing the risk of complications, reducing the cost to patients and medical facilities and improving overall patient outcomes," said Jack McCarthy, vice president of U.S. healthcare sales at Z-Medica. "We are very pleased to be working with the experienced HealthTrust team in this important effort to bring QuikClot products to more healthcare providers and patients who can benefit from them."
QuikClot products are impregnated with a mineral called kaolin that has been clinically shown to accelerate the body's natural coagulation cascade, helping healthcare professionals, first responders, law enforcement officers, consumers and adventure and outdoor sports enthusiasts rapidly control bleeding. Kaolin is a naturally-occurring, inorganic mineral that does not contain any botanicals, biological material or shellfish products and does not cause any exothermic reaction or vascular complications. QuikClot products are credited with helping thousands of people survive traumatic blood loss every year.
"Controlling bleeding in a hospital setting is essential for minimizing the length of procedures, reducing the risk of complications, reducing the cost to patients and medical facilities and improving overall patient outcomes," said Jack McCarthy, vice president of U.S. healthcare sales at Z-Medica. "We are very pleased to be working with the experienced HealthTrust team in this important effort to bring QuikClot products to more healthcare providers and patients who can benefit from them."
QuikClot products are impregnated with a mineral called kaolin that has been clinically shown to accelerate the body's natural coagulation cascade, helping healthcare professionals, first responders, law enforcement officers, consumers and adventure and outdoor sports enthusiasts rapidly control bleeding. Kaolin is a naturally-occurring, inorganic mineral that does not contain any botanicals, biological material or shellfish products and does not cause any exothermic reaction or vascular complications. QuikClot products are credited with helping thousands of people survive traumatic blood loss every year.
Subscribe to:
Posts (Atom)


