| A type of artificial platelet being developed to help natural blood platelets form clots faster offers promise for saving the lives of soldiers, as well as victims of car crashes and other severe trauma. | |
| In preclinical tests led by a Case Western Reserve University researcher, the artificial platelets, called "hemostatic nanoparticles," when injected after blast trauma dramatically increased survival rates and showed no signs of interfering with healing or causing other complications weeks afterward. | |
| "The nanoparticles have a huge impact on survival—not just in the short term, but in the long term," said Erin Lavik, an associate professor of biomedical engineering at Case Western Reserve. Other researchers had raised concerns that the foreign matter would interfere with healing, or form free-floating clots, but "we saw none of that." | |
| The research, published in the Proceedings of the National Academy of Sciences this week ("Intravenously administered nanoparticles increase survival following blast trauma"), show the survival rate of mice models of blast trauma treated with the nanoparticles increased to 95, compared to 60 percent for those untreated. | |
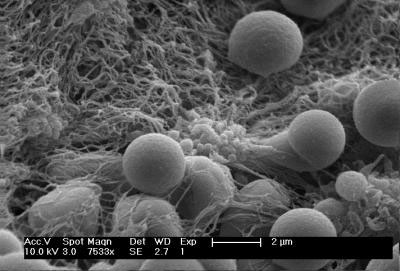 | |
| Spherical hemostatic nanoparticles accumulate on a clot-stabilizing mesh of fibrin the body produces. (Image: Andrew Shoffstall) | |
| Also, no unwanted side effects, such as accumulation of the nanoparticles, clot formation or aberrant healing, were found during examinations one ands three weeks after the injection. | |
| Lavik worked with Margaret M. Lashof-Sullivan, Erin Shoffstall and Kristyn T. Atkins, of Case Western Reserve; Nickolas Keane and Cynthia Bir of Wayne State University and Pamela VandeVord of Virginia Tech. | |
| Explosions account for 79 percent of combat-related injuries and are the leading cause of battlefield deaths, according to researchers at Veterans Affairs hospitals and the federally run Uniformed Services University of the Health Sciences. | |
| The primary blast wave, flying shrapnel and being thrown to the ground cause the lungs, liver, kidneys and other organs to hemorrhage and bleed uncontrollably. | |
| Such uncontrolled bleeding from collisions, blows and falls is also the leading cause of death among victims age 5 to 44 in the United States. | |
| Natural blood platelets are the key ingredient to stopping bleeding, a process called hemostasis. The process works well for typical cuts and scrapes, but can be overwhelmed with serious injuries. | |
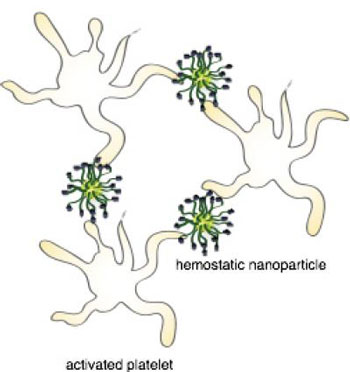 | |
| This is a schematic of hemostatic nanoparticles linking with blood platelets. The nanoparticles significantly increased survival rate from blast trauma in preclinical testing. (Image: Erin Lavik) | |
| Hospitals try to stem internal bleeding by giving trauma patients blood products or the hemophilia medicine called recombinant factor VIIa, but there isn't a good option for the battlefield or accident scenes. Recombinant factor VIIa must be refrigerated, costs up to tens of thousands of dollars per treatment and can cause clots in brain and spinal cord injuries, which are common from explosions. | |
| Lavik's team has fine-tuned the nanoparticles to increase clotting efficiency. "They are incredibly simple… spheres with arms of peptides that react with activated blood platelets in damaged tissues to help clots form more quickly," she said. | |
| The particles are made from short polymer chains already approved for other uses by the U.S. Food and Drug Administration. In earlier testing, rat models injected with the nanoparticles stopped bleeding faster than untreated models. | |
| The dry particles remained viable after two weeks on a shelf. A medic in the field or an ambulance crew would add saline, shake and inject them, the researchers say. | |
| Further research and testing are underway. Clinical trials on humans are likely at least five years out, Lavik said. |
Monday, June 30, 2014
Clot-building nanoparticles raise survival rate following blast trauma Read more: Clot-building nanoparticles raise survival rate following blast trauma
Sunday, December 11, 2011
Z-Medica Donates $1.5 Million in QuikClot
WALLINGFORD, Conn., Dec 08, 2011 (BUSINESS WIRE) -- Z-Medica Corporation, a medical device company which develops and distributes innovative hemostatic agents, announced that it has donated $1.5 million in QuikClot(R) the company's life-saving hemostatic agents, to AmeriCares, a nonprofit global health and disaster relief organization. The QuikClot products will be used by AmeriCares for disaster relief efforts as well as its ongoing health programs around the globe, helping to prevent loss of life and limb. Z-Medica had previously donated $150,000 of its QuikClot products to AmeriCares for use in Haiti following the earthquake there in 2010.
"We had great success providing QuikClot for use in Haiti following the earthquake and for other relief efforts and we are grateful that Z-Medica has responded to our request for an additional donation of this life-saving tool," said Dr. Frank J. Bia, AmeriCares Medical Director. "QuikClot is now a standard part of our response efforts and we make sure it is part of our aid deliveries for disasters and our ongoing partnerships."
QuikClot is an ideal product for use in the field, including disaster relief areas or by first responders, due to its ease-of-use and ability to achieve hemostasis in as little as three minutes. The United States Department of Defense currently uses QuickClot Combat Gauze(TM) as the official first-line hemostatic treatment for traumatic bleeding in all branches of the United States military. QuikClot is also regularly used by first-responders and medical professionals in the field and in hospital clinical environments such as the cardiac catheterization laboratory, interventional radiology, the emergency room and virtually anywhere else where bleeding needs to be controlled.
"AmeriCares is an extremely professional and efficient organization and we have the highest confidence that they will save many lives with this donation," said Brian Herrman, Chief Executive Officer, Z-Medica. "Every US soldier, marine, and airman carries QuikClot at all times, which has saved many lives. Our mission at Z-Medica, therefore, is to ensure that all medical professionals and first responders everywhere have access to QuikClot, in both disaster areas, as well as in day-to-day situations such as hospital trauma units, emergency rooms and the cardiac catheterization labs."
Tuesday, March 1, 2011
US ARMY STUDY SHOWS STB LIFESAVING TECHNOLOGY'S®FAST® DRESSING SUPERIOR FOR CONTROLLING ARTERIAL BLEEDING.
The study compared currently available agents with the FAST® dressing, in a coagulopathic hemorrhage model simulating a gunshot wound to a major peripheral artery. The FAST® dressing produced significantly better outcomes (higher survival rate, longer survival time, higher incidence of stable hemostasis and less blood loss). STB®'s FAST® dressing was the only agent that stopped bleeding and prevented exsanguination in most subjects.
"The consistently better results obtained using the FAST® Dressing reflect the results of applying the unique FAST® technology to this most challenging bleeding problem. The ability to optimally mix the fibrinogen and thrombin components during manufacture results in a hemostatic product of unprecedented effectiveness, flexibility and ease of handling. This technology is unaffected by the coagulation state of the patient and therefore functions when other types of products cannot. This has significant implications for the treatment of both military and civilian casualties," commented Dr. Martin MacPhee, STB®'s Chief Scientific Officer.
The report emphasizes the urgent need for methods such as this to control hemorrhage in the field and operating rooms to potentially reduce war mortality, as uncontrolled bleeding remains the leading cause of potentially preventable death in combat casualties. Hemorrhage is also a leading cause of death in civilian trauma patients.
"Study after study has confirmed the faith we have in our product's ability to save lives, and it's why we refer to our technology as 'A Breakthrough for Life.' Test results such as this recent report demonstrate why the Army continues to support for our research, assisting us in accelerating the step-up to cGMP manufacturing, and the necessary work required to file for FDA approval to initiate human clinical trials," said Richard Moscarello, STB®'s CEO.
Tuesday, November 16, 2010
Blood-clotting drug given to wounded soldiers can cause heart attacks
The treatment is used to stop serious bleeding in injured troops, but trials show the drug increases the risk of blood clots forming in arteries, which can kill or cause complications that result in amputation.
The dangerous side effects are all the more concerning because years of trials have yet to prove the drug is any better at saving the lives of injured soldiers than a placebo.
The drug, called NovoSeven, was licensed more than a decade ago to stop bleeding in haemophiliacs, but is used by military doctors and in civilian hospitals on an "off-label" basis to treat patients suffering from major blood loss due to trauma or surgery. The drug, also known as recombinant factor seven, costs several thousand pounds per patient but an effective alternative called tranexamic acid costs just £5 per patient.
A spokesman for the Ministry of Defence confirmed that NovoSeven was used by UK forces as "a medicine of last resort", when all other attempts to stem bleeding had failed. The US military also uses the drug.
Prof Ian Roberts, an expert in trauma care at the London School of Hygiene and Tropical Medicine, warned in 2006 that NovoSeven was being used off-label before trials had clarified whether or not it helped save lives.
"There are both civilian patients and wounded soldiers who will have been given this drug and the best evidence shows they would not have benefited, but would have experienced heart attacks, strokes and possible amputations," Roberts said.
By continuing to use the drug, the military and hospital surgeons were leaving themselves vulnerable to legal action, he added. "You cannot defend giving this treatment outside of a randomised controlled trial," he said.
He added that the US military, which has used NovoSeven for years, should explain why it embraced the drug. "What advice were they acting on? Was the advice they received truly independent, or were people compromised in any way? It is important to know," he said.
Dr Mads Krogsgaard Thomsen, chief science officer at Novo Nordisk, the Danish company that makes NovoSeven, said the drug posed very low risk when used under licence to stop bleeding in haemophiliacs and for certain rare blood clotting disorders. "We cannot encourage as a company, by any means, the off-label use of NovoSeven. This is not something we are promoting and it is not something we are responsible for."
Thomsen estimates that between 10% and 20% of NovoSeven is used off-label. "Every now and then, physicians do use it as a last resort in patients that are otherwise likely to die," he said.
Roberts believes the case for using NovoSeven is weakened further by the availability of a much cheaper alternative drug, tranexamic acid, which is known to save lives in bleeding patients without dangerous side effects. A trial of tranexamic acid, called Crash-2, was published in the Lancet in June.
Marcel Levi, professor of internal medicine at Amsterdam Medical Centre, published a review of NovoSeven trials in the New England Journal of Medicine last week that was funded by NovoNordisk. "If you look at whether the drug stops bleeding, or whether fewer transfusions are needed, then many studies are positive. If the only thing that matters is mortality, then it is much harder to prove that this is a useful drug," he said.
Beverley Hunt, a consultant haematologist who worked on the Crash 2 trial at Guy's and St Thomas's Hospital in London, said: "The issue around the use of factor seven in patients with massive blood loss is that there is a lack of evidence to show us how clinically efficacious it really is, what dose to use and when to use it. The problem with using an agent that alters blood clotting is that it is a tightrope walk in reducing the risk of bleeding without increasing the risk of blood clots.
"The recent data showing that its use is associated with a 5% risk of arterial thrombosis means we should not be using it early in the management of massive blood loss. If however despite normal good practice, somebody is bleeding to death and you have nothing left, are you willing to take a 5% risk of arterial thrombosis, and the answer is yes you are."
Tuesday, September 21, 2010
Z-Medica Signs Distribution Agreement With Biomedica
QuikClot® products received CE Mark from the European Union in November 2009 and the company has been negotiating distribution agreements with a series of best-of-breed medical device distributors such as Biomedica in European markets since then.
“We are very pleased that we have signed this distribution agreement with Biomedica because of their strong relationships, local sales presence and customer support in Austria, Hungary and Switzerland,” said Brian Herrman, Chief Executive Officer, Z-Medica. “Their focus on cardiology, orthopedics and trauma will be key to getting QuikClot into the hands of surgical practitioners and first responders for the first time in this region, so that they and their patients can benefit from QuikClot’s ability to achieve hemostasis safely and rapidly.”
QuikClot is a surgical gauze impregnated with kaolin, an inert mineral with no known contraindications, and can achieve hemostasis in severe bleeding situations in as little as three minutes. QuikClot is widely used throughout several clinical specialties, including cardiology, interventional radiology, critical care, dermatology, emergency medicine, orthopedics and OB/Gyn, and after months of testing against 12 other hemostatic products in the marketplace, the military version of the kaolin gauze (“Combat Gauze”) was chosen as the exclusive product for use by all US Military Forces in 2008. It continues to be the exclusive product used by all USA military forces for first line treatment of bleeding hemorrhage.
“We are very pleased to add QuikClot to our product portfolio,” said Dr. Stefan Marenzi, Managing Director of Biomedica. “We believe that the product will be well-received by our clients across several different specialties.”
Saturday, September 18, 2010
HemCon Medical Technologies Will Appeal Patent Judgment
Marine Polymer sued HemCon in 2006, alleging that HemCon had violated its patent covering a biocompatible chitosan compound. Marine Polymer’s patent describes a chitosan compound that is derived from the sterile culturing of marine micro algae.
HemCon uses a chitosan compound to manufacture highly effective bandages that have been used in battlefield conditions by the U.S. military, among others. HemCon does not use chitosan that is derived from sterile culturing of micro algae, as described in the Marine Polymer patent.
HemCon has separately initiated a proceeding to reexamine the validity of the patent through the US Patent & Trademark Office. In 2009, HemCon filed a request with the Patent Office to reexamine, and possibly invalidate or limit, Marine Polymer’s patent in light of prior publications about chitosan. The Patent Office granted the Request for Reexamination in November 2009. On April 1, 2010, the Patent Office issued a first office action, rejecting all claims of Marine Polymer’s patent. Marine Polymer has filed a response canceling some patent claims and arguing that the remaining claims are valid as originally issued.
Wednesday, September 15, 2010
Z-Medica launches new trauma kit
 WALLINGFORD, Conn., Sept. 15 (UPI) -- A new hemostatic trauma kit to quickly stop bleeding is now available to law enforcement agencies around the United States.
WALLINGFORD, Conn., Sept. 15 (UPI) -- A new hemostatic trauma kit to quickly stop bleeding is now available to law enforcement agencies around the United States.Z-Medica, which has headquarters in Connecticut, this week launched its QuikClot Belt Trauma Kit designed for law enforcement agencies and said it can achieve hemostasis in severe bleeding situations in as little as three minutes.
"We understand that law enforcement officers often face situations that involve traumatic bleeding where emergency medical care is not available, so we designed a practical tool to help them almost immediately stop uncontrolled bleeding," said Z-Medica Chief Executive Officer Brian Herrman. "The new QuikClot Belt Trauma Kit includes the QuikClot product that every U.S. soldier carries, and which many law enforcement officers are already carrying, combined with all of the tools needed to stop traumatic bleeding in a lightweight pack."
The 5.75-inch-by-4-inch kit includes either QuikClot First Response or QuikClot Combat Gauze, plus SWAT-Tourniquet, a cardiopulmonary resuscitation shield and gloves. The kit is designed to be worn on an officer's duty belt along with other gear, taking up little space.
Monday, August 30, 2010
QuikClot goes Japanese
 WALLINGFORD, Conn.--(BUSINESS WIRE)--Z-Medica Corporation, a medical device company developing innovative hemostatic agents, today announced that it has signed an exclusive distribution agreement with Nihon-Kohden, Japan's leading manufacturer, developer and distributor of medical electronic equipment . The agreement allows Nihon-Kohden the exclusive right to distribute Z-Medica’s QuikClot line of hemostatic agents to hospital, military and law enforcement markets throughout Japan. The companies have been working together since early 2009 to obtain regulatory approval. Such approval for the sale of Z-Medica’s full line of QuikClot hemostatic gauze products was approved by the Japanese Ministry of Health, Labor and Welfare in March 2010.
WALLINGFORD, Conn.--(BUSINESS WIRE)--Z-Medica Corporation, a medical device company developing innovative hemostatic agents, today announced that it has signed an exclusive distribution agreement with Nihon-Kohden, Japan's leading manufacturer, developer and distributor of medical electronic equipment . The agreement allows Nihon-Kohden the exclusive right to distribute Z-Medica’s QuikClot line of hemostatic agents to hospital, military and law enforcement markets throughout Japan. The companies have been working together since early 2009 to obtain regulatory approval. Such approval for the sale of Z-Medica’s full line of QuikClot hemostatic gauze products was approved by the Japanese Ministry of Health, Labor and Welfare in March 2010.“We are very pleased that we have signed this distribution agreement with Nihon-Kohden because of their longstanding positive reputation and vast reach throughout the Japanese market,” said Brian Herrman, Chief Executive Officer, Z-Medica. “We believe that clinical practitioners outside of the US will find QuikClot to be a useful tool in achieving hemostasis in a fast, safe and convenient manner, and we intend to continue expanding our international distribution through best-of-breed partners such as Nihon-Kohden.”
QuikClot is a surgical gauze impregnated with kaolin, an inert material with no known contraindications, and can achieve hemostasis in severe bleeding situations in as little as three minutes. QuikClot is widely used throughout several clinical specialties, including cardiology, interventional radiology, critical care, dermatology, emergency medicine, orthopedics and OB/Gyn, and after months of testing against 12 other hemostatic products in the marketplace, the military version of the kaolin gauze (“Combat Gauze”) was chosen as the exclusive product for use by all US Military Forces in 2008. It continues to be the exclusive product used by all USA military forces for first line treatment of bleeding hemorrhage.
“We are very pleased to have this opportunity to market in Japan QuikClot which helps many patients who suffer from bleeding,” said Hiroshi Aida, General Manager of Import Business Operation, Nihon Kohden. “QuikClot also helps medical professionals with much shorter time for achieving hemostasis. It is a nice addition for our product portfolio.”
Z-Medica and Nihon-Kohden have been working together since early 2009 in order to gain the recently granted regulatory approval. The QuikClot line of products to be distributed by Nihon-Kohden in Japan include:
QuikClot Combat Gauze is a soft, white, sterile, nonwoven 3” by 12 feet rolled or z-folded gauze impregnated with kaolin. Each roll of QuikClot Combat Gauze is individually wrapped in an easy rip, military grade foil pouch. Indicated for temporary external control of traumatic bleeding, QuikClot Combat Gauze is flexible and pliable and contours to all wounds. Recommended as the number one hemostatic agent by the COTCCC (Committee on Tactical Combat Casualty Care Committee), QuikClot Combat Gauze is the only product carried by all branches of the US Military to control life-threatening hemorrhage.
QuikClot Emergency Dressing is an easy-to-use dressing which can achieve hemostasis in as little as three minutes, helping to prevent loss of life and limb. Because QuikClot requires very little training to administer, it can be effectively used by medical personnel, civilian first-responders and medical professionals in the field and in clinical environments. This dressing comes in various sizes, including a 4-ply 4” by 4” (10cm x 10cm) and a 6-ply 2”x2” (5cm x 5cm) format.
QuikClot Interventional Hemostatic Bandage consists of a soft, white, double sterile, hydrophilic pad impregnated with kaolin. It is double-wrapped in a blister package and foil pouch for aseptic technique. QuikClot Interventional Hemostatic Bandage is applied topically as an adjunct to manual compression and is indicated for the local management and control of external bleeding from vascular access sites and percutantous catheters or tubes utilizing sheaths up to 12 Fr.
QuikClot Trauma Pad consists of a soft, white, double sterile, three-ply 12”x12” (30cm x 30cm) pad impregnated with kaolin. It is double-wrapped in a peelable foil package for aseptic technique. QuikClot Trauma Pad is indicated for temporary external use to control traumatic bleeding and is also x-ray detectable to ensure proper removal.
About Z-Medica
Z-Medica Corporation is a medical device company developing innovative hemostatic agents. The company manufactures and markets its QuikClot® family of products for hemostasis for use by healthcare professionals, first responders, law enforcement officers and the military. QuikClot products rapidly enhance the body’s natural coagulation process, helping to achieve hemostasis faster. Z-Medica’s QuikClot® Combat Gauze™ product was chosen by the Committee on Tactical Combat Casualty Care (TCCC) as the United States Military’s sole source supplier for first-line hemostatic treatment, based on tests conducted by the Naval Medical Research Center and the U.S. Army Institute for Surgical Research. It continues to be the exclusive product used by all USA military forces for first line treatment of bleeding hemorrhage. QuikClot® products are also widely used by first responders, in hospital emergency rooms, interventional cardiology and radiology laboratories and other healthcare environments where bleeding requires fast and effective control. Z-Medica, named one of the Top 100 Technology “Companies to Watch” in 2008 by the Connecticut Technology Council, is a privately-held company based in Wallingford, CT. More information about Z-Medica Corporation is available at www.z-medica.com.
About Nihon Kohden
Nihon Kohden is Japan’s leading manufacturer, developer and distributor of patient monitors, defibrillators, ECGs, EEGs, EP/EMGs, hematology analyzers and other medical electronic equipment, with subsidiaries in the United States, Europe, and Asia, and distributors in nearly every country in the world. Nihon Kohden is a publicly held company listed in the First Section of the Tokyo Stock Exchange. In addition to designing medical equipment for hospital and clinic use, Nihon Kohden actively contributes to the advance of medical technology.
Saturday, August 28, 2010
US Military faces challenges from Chinese Knock-offs
 As anyone who has served in combat knows, if a buddy is wounded, the first two things you need to do are make sure he can breathe and his bleeding is stopped.
As anyone who has served in combat knows, if a buddy is wounded, the first two things you need to do are make sure he can breathe and his bleeding is stopped.For the past several years, troops serving in Afghanistan and Iraq have used an advanced Combat-Application-Tourniquet (C-A-T) developed by Composite Resources in Rock Hill, S.C. The tourniquet features a nylon strap and a plastic rod to tighten the strap to stop bleeding.
The regulation C-A-T costs about $28. But about two years ago the Army detected
 cheap knock offs made by a Hong Kong company that had entered the military's supply chain in Afghanistan and Iraq. The imitation sold for about $8.50.
cheap knock offs made by a Hong Kong company that had entered the military's supply chain in Afghanistan and Iraq. The imitation sold for about $8.50.They're accurate looking fakes, right down to the label and national stock number.
But as Col. John Kragh, a doctor at the U.S. Army Institute of Surgical Research at Fort Sam Houston, pointed out in June, the rod on the fake tourniquet "is bendable to a point where it cannot work right. It's like bending Gumby's arm."
He said the fake tourniquet could be fatal because it cannot stop bleeding. Kragh added a
 decentralized ordering system probably accounts for the presence of the fake tourniquets in the field, with low-level supply personnel ordering the knock offs over the Internet based on price.
decentralized ordering system probably accounts for the presence of the fake tourniquets in the field, with low-level supply personnel ordering the knock offs over the Internet based on price.The Defense Department issued a warning about the knock-offs in April, Kragh said, and the Food and Drug Administration this month put out a safety alert about the tourniquets, which are also used by civilian first responders.
The lesson here is a good deal isn't always that; it can even be deadly.
Poll Result - Which Geographical Manufacturing Area for Hemostatic Devices Causes Concern?
Controversies
2007 Chinese Export Recall
The 2007 Chinese export recalls were a series of scandals involving tainted food and products exported from China, starting with tainted pet food imported from China to the United States that poisoned pets. The recalls sparked international concern that many products made in China do not meet minimum quality standards. Soon after, the US halted imports of seafood from China after tests detected the presence of drugs unapproved in the US.China has gone on record to admit that nearly a fifth of products made in China do not reach minimum standards. Also, some children's toys made in China were found to contain excessive levels of lead, prompting widespread concern. In 2006, shipments of cough syrup and other medicines, imported from China to Panama and laden with the toxin diethylene glycol, caused mass poisonings and killed 83 people.
On December 19, 2007, The US House of Representatives passed legislation (H.R. 4040) that would significantly amend the current U.S. safety establishment for consumer products imported from China.
2008 Chinese heparin and milk scandals
In March 2008, major recalls of heparin were announced by the U.S. Food and Drug Administration (FDA) due to contamination of the raw heparin stock imported from China.
The raw material for the recalled heparin batches was processed in China from pig's intestines by the American pharmaceutical firm Scientific Protein Laboratories. The U.S. Food and Drug Administration was quoted as stating that at least 81 deaths were believed linked to a raw heparin ingredient imported from the People's Republic of China, and that they had also received 785 reports of serious injuries associated with the drug’s use. According to the New York Times, "Problems with heparin reported to the agency include difficulty breathing, nausea, vomiting, excessive sweating and rapidly falling blood pressure that in some cases led to life-threatening shock."
Upon investigation of these adverse events by the FDA, academic institutions, and the involved pharmaceutical companies, the contaminant was identified as an "over-sulphated" derivative of chondroitin sulfate, a popular shellfish-derived supplement often used as a treatment forarthritis. Since over-sulphated chondroitin is not a naturally occurring molecule, costs a fraction of true heparin starting material, and mimics the in-vitro properties of heparin, the counterfeit was almost certainly intentional as opposed to an accidental lapse in manufacturing. The raw heparin batches were found to have been cut from 2-60% with the counterfeit substance, and motivation for the adulteration was attributed to a combination of cost effectiveness and a shortage of suitable pigs in China.
When the FDA conducted an inspection of Baxter's Chinese Heparin supplier, it found serious deficiencies at the facility which the FDA detailed in a warning letter.
The FDA has stated that it does not have the funds nor bear the responsibility to inspect on a regular basis overseas manufacturers of active pharmaceutical ingredients such as heparin.
In November 2008, the FDA seized eleven lots of heparin from Celsus Laboratories Inc., a manufacturer in Cincinnati, Ohio.
One year later, the 2008 Chinese milk scandal refers to a food safety incident in mainland People's Republic of China involving milk and infant formula which had been adulterated with melamine, an organic base combined with formaldehyde to form plastic. The result was catastrophic - By 22 September, nearly 53,000 illnesses, over 12,800 hospitalizations, and four infant deaths had been reported, caused by kidney stones and other renal failure.The chemical appeared to have been added to milk in order to cause it to appear to have a higher protein content. The same chemical was also involved in a series of pet food recalls in 2007. In November 2009, two individuals were executed for endangering public safety and producing and selling toxic food.
2009 Made in India scandal
In June 2009, the Nigerian Government Drug Regulatory Authority (NAFDAC) reported about the detention of a large consignment of fake anti-malarial generic pharmaceuticals labeled Made in India but produced in China. The Laboratory analysis conducted by NAFDAC revealed the drugs to be fake and had it not been intercepted, about 64,200 adults would have been affected. The consignment containing Maloxine and Amalar tablets, used for the treatment of Malaria, were valued at 32.1 million Naira and were produced, packed and shipped from China. After Indian Authorities took up the matter, The State Food and Drug Administration (SFDA) of China was asked to investigate the matter[10]. In December 2009, 6 chinese traders were sentenced to death for their involvement.
In July 2009, customs officials in the South Indian port city of Chennai seized spurious cosmetics and mobile phone batteries worth Rs. 30 million, imported into India from Scheko in China. The 40 foot container was held up at the port for more than 75 days and opened on 13th July when the importer did not file a bill of entry. It was reported to contain over 187,000 batteries with Nokia holograms and stickers, and 126,000 cosmetic items including face packs, lipsticks and hair gels.
Sunday, August 22, 2010
‘War is the only proper school for surgeons’
 It was September 2005, and Meghoo, now a lieutenant colonel, had just completed his surgical residency. Trained in repairing the car crash injuries and the occasional gunshot wound that trickled into his Texas emergency room, he now was confronted with the devastating and hugely varied wounds of modern warfare: legs and arms mangled by explosions, arteries severed by bullets, vital organs peppered with shrapnel.
It was September 2005, and Meghoo, now a lieutenant colonel, had just completed his surgical residency. Trained in repairing the car crash injuries and the occasional gunshot wound that trickled into his Texas emergency room, he now was confronted with the devastating and hugely varied wounds of modern warfare: legs and arms mangled by explosions, arteries severed by bullets, vital organs peppered with shrapnel.“It was a bit overwhelming,” he recalled, “but then you think to yourself, ‘Wow, this doesn’t even exist in busy civilian hospitals. You need to be in a war zone to see this.’ ”
The ancient Greek physician Hippocrates said that “war is the only proper school for surgeons,” and the war in Iraq has been just that, but with an important development: Lessons were learned and shared immediately.
In the past, doctors had to wait until the gunfire stopped before examining what lessons had emerged.
There are dozens of volumes just about World War II, published long after the war, said Air Force Dr. (Lt. Col.) Raymond Fang, trauma director Landstuhl Regional Medical Center in Germany.
But in Iraq, improvising surgeons and up-to-the minute observational research have resulted in techniques that helped troops survive at a greater rate than in any previous war. Some are high-tech and novel; others were rediscovered; and a few are reinterpretations of old protocols. Moreover, these lessons were documented and shared as the war raged.
Military surgeons have been able to rewrite the book on trauma before U.S. troops have even left the country. “War Surgery in Afghanistan and Iraq: A Series of Cases, 2003-2007” was published by the U.S. Army in 2008, detailing 83 cases from 53 battlefield
doctors.
Now the lessons and techniques tested in Iraq are being honed in Afghanistan, and new ones are being explored.
“It’s not about the next war anymore,” Meghoo said, who recently completed his second tour at Forward Operating Base Sharana near the Afghanistan-Pakistan border.
The advances are needed now.
Wars have always birthed medical advancements.
During the Civil War, physicians found new ways to amputate limbs and began using rags soaked in chloroform to anesthetize patients. The typhoid vaccine was developed during World War I, when an Army officer also established the first blood depot. World War II brought the mass use of penicillin, and the Korean War, helicopter evacuation.
One of the legacies of Iraq is a new emphasis on stopping blood loss. Better body armor and helmets protected troops’ vital organs, but high-velocity bullets, rocket-propelled grenades and roadside bombs inflicted the exposed limbs with massive, bleeding wounds.
To stanch the bleeding, doctors turned to one of the oldest and simplest tools in battlefield medicine: the tourniquet. Tourniquets had been maligned and shunned since World War II because of fears that they caused gangrene and limb loss. They do, when used for long periods — but faster evacuation and hospitalization combined with tourniquets saved hundreds of lives in Iraq. A 2008 review of records at the 31st Combat Support Hospital in Baghdad showed that four in seven deaths could have been prevented had a tourniquet been used before the patient arrived.
“This is a simple mechanical thing done since the time of the Egyptians,” Fang said, “and now it’s returning.”
Soldiers in the field are issued one combat application tourniquet, or CAT; some carry more than half a dozen. Slipped around a limb, the tourniquet’s nylon strap is cinched, and the plastic bar on top is twisted, crimping the artery below. The lever is cranked with one hand, so servicemembers can secure their own tourniquets even when badly injured.
Sgt. Chris Bickford, a combat medic who has deployed to Iraq twice, said he trains all his soldiers to clamp on a tourniquet in less than 12 seconds.
“Before we could only use pressure dressings, and there was the chance you may lose the life,” he said. “Now the way the tourniquets are built, allowing them to be placed high on the limb — that saves lives.”
At the start of the Iraq war, innovative clotting agents such as QuikClot were also poured into open wounds. QuikClot, a loose substance made of synthetic absorbents that looks like dry clay, draws water from blood, helping it coagulate. But surgeons often had to pick out the sticky granules, and in some cases QuikClot burned tissue, or even the eyes of the patient on a windy day.
QuikClot’s manufacturer, Z-Medica Corp., soon replaced the loose product with a bag filled with the clotting agent, then, finally, combat gauze treated with it. Medics in the field now push the gauze into wound cavities. The gauze is “easier to carry and much more comfortable for the patient,” Bickford said.
 Now the military is looking for alternatives to these synthetic materials, Fang said. One possibility is dried plasma, the liquid part of blood that has proteins and clotting factors. The dried plasma would be revived in a saline solution and then given to wounded troops intravenously or injected into the bone marrow. The latter technique keeps medics from having to search for a vein, a difficult task when soldiers are covered in thick packs and bleeding, said Col. Dallas Hack, director of the Combat Casualty Care Research Program.
Now the military is looking for alternatives to these synthetic materials, Fang said. One possibility is dried plasma, the liquid part of blood that has proteins and clotting factors. The dried plasma would be revived in a saline solution and then given to wounded troops intravenously or injected into the bone marrow. The latter technique keeps medics from having to search for a vein, a difficult task when soldiers are covered in thick packs and bleeding, said Col. Dallas Hack, director of the Combat Casualty Care Research Program.“The bone marrow is a fairly large area,”” Hack said, “and you can do it by feel.”
Clinical trials of two forms of dried plasma, one freeze-dried and the other spray-dried, are under way, and it could be in the hands of medics within three to five years, Hack said.
Fang said long-held conventions about blood transfusions also changed. Doctors once pumped saline into hemorrhaging patients to restore hydration, and then added red blood cells and extra platelets. Now surgeons try to “emulate whole blood,” Fang said, forgoing saline and pumping in equal amounts of platelets, cells and plasma.
Before the war, surgeons had regarded the freshness of donor blood as unimportant — as long as it was used within a two-month window. But downrange doctors discovered that fresher red blood cells carry more oxygen, which keeps vital organs working better and provides more oxygen to the brain, helping prevent comas.
“There is a push downrange to use blood that is 14 days old or younger,” Fang said.
At forward operating bases in Iraq, loudspeakers often called for blood donors during periods of heavy fighting. Troops lined up at combat hospitals to donate as the wounded were carried in.
It’s a procedure that has continued in Afghanistan, Meghoo said, even at small outposts.
“You’d put the call out,” he said, “and people would just descend.”
Surgeons like Meghoo also practiced closer to the battlefield than in previous wars, often in the dusty tents of forward operating bases. There, teams lacked many resources of typical trauma hospitals, including imaging equipment such as CAT scanners and fluoroscopes. But they performed minimal yet crucial operations to stabilize a patient, often within the critical “golden hour” after injury, said Dr. (Col.) David P. Blake, director of trauma at the Balad hospital. After the initial lifesaving surgery, patients were taken to Balad, where they were warmed up and given fluids, allowing them to recover enough for the next surgery.
“You control what is immediately going to kill them,” Blake said, “and then you come back another day to address the details.”
Roadside bombs mangled limbs, leaving many soldiers amputees. To prevent this, surgeons improvised with shunts, tiny plastic tubes placed into damaged blood vessels. The combat surgeons found the shunts, which had been used occasionally by vascular surgeons, indispensable to keep blood flowing to troops’ injured limbs, hands or feet until the damaged vessels could be repaired.
Meghoo, for example, had a patient in Afghanistan who was shot in the leg by a high-velocity bullet. The patient’s femoral artery had been severed, and worse, the bullet had burned the edges of the artery, causing it to contract.
“That’s a huge hole,” he said, showing a picture of the gaping wound. “And I can’t sew the [artery] back together because the ends don’t reach anymore.”
To control the flow of blood, Meghoo placed two shunts into the patient’s leg, one in the artery and the other in the vein, and sent him on to another hospital for further care.
In previous wars, Meghoo said, he likely would have bled to death or lost a limb.
Devices developed at civilian hospitals were also brought to the battlefield, where doctors found new uses for them.
Before 2004, doctors opened and cleared wounds of blood-soaked gauze and other debris twice daily, a treatment that was painful for patients, but needed to keep their wounds clean. The frequent changing of dressings was common practice for centuries, Fang said, until surgeons at Balad experimented with a vacuum that sucks away the excess fluid, allowing a wound to be sealed for nearly three days.
With negative pressure wound therapy, the wound cavity is covered with a clear plastic bandage with a suction tube attached, then vacuum-sealed. Designed for chronic wounds, the suction helps troops’ tissues heal faster “because all that wound soup is not staying around,” said Lt. Col. Joe Sniezek, director of the Seventh Joint Combat Casualty Research Team.
Better still, patients on long evacuation flights no longer need to have their bloody dressings removed and cleaned in transit.
“It remains to be seen whether the outcome is better,” said Fang, “but it definitely makes it more comfortable for the patients.”
Several high-tech devices were tested for the first time on troops, including an infrared camera that helps doctors detect compartment syndrome, a crimping of the blood vessels in the leg or arm. Deep wounds cause muscle inside the limb to swell, potentially leading to gangrene. The only way to relieve the pressure, or even diagnose compartment syndrome, is to flay the leg or arm muscles open.
The surgery works, said Sniezek, but it’s a “morbid procedure, making an incision all the way down the limb.”
The infrared device, however, allows doctors to detect any difference between the temperatures of the body’s core and the limbs, an indicator that blood is not flowing correctly. If the temperatures are within normal range, surgeons can potentially avoid an unnecessary opening of the limb.
“This is a nice example of how new technologies are being integrated here on the battlefield,” said Sniezek, “and the study is now moving to Afghanistan.”
A cardiothoracic surgeon at Landstuhl was the first to save a patient with the Novalung — a simple-looking slender box with a complex membrane inside that acts as a temporary lung. It connects to blood vessels in the groin, allowing blood to flow from one leg into the box, where it passes through a filter that leaches off the carbon dioxide and infuses the cells with oxygen, mimicking the trade-off that takes place naturally in the lungs. The refreshed blood then returns to body through the other leg.
Unlike a heart-lung machine, the Novalung doesn’t require a mechanical pump because it “works off the body’s own blood pressure,” Fang said.
Approved by European regulators but not yet by the U.S. Food and Drug Administration, the Novalung has been used successfully on about a dozen troops transported to Landstuhl, Fang said. But each time they use it, doctors must submit to the FDA that it is being used to save a life.
“Every time there is a war we learn a lot, because we have to,” said Fang. “And what you learn over years of time is condensed.”
Meghoo recalled using the many lessons that he learned in Iraq during his recent deployment to FOB Sharana, where he was one of two surgeons working in a shed.
“It could be a very lonely feeling,” he said of being away from the safety net of a large hospital with many specialists. “You can’t pass things off to anyone else, and these people have big problems, and you are the only one who can fix them.”
On one occasion an Afghan woman had her right shoulder severed in a mortar attack. Blood gushed from the wound, making it impossible for Meghoo to reach the artery.
“It’s the equivalent of a fire hydrant,” he said. “You can’t go through the water to find the hole. You have to know where the shut-off valve is.”
Meghoo removed part of the woman’s collarbone, and then stemmed the flow of blood by temporarily cinching the artery.
“I remember that case,” he said, “because it was a skill I didn’t have until after I deployed to Iraq.”
The techniques discovered by military surgeons are trickling down to civilian hospitals as well, said Dr. James Dennis, a visiting vascular surgeon at Landstuhl who choked up while talking about the troops with catastrophic injuries whom he had treated in the intensive care unit.
“You can’t help but be moved by what these young people are going through,” said Dennis, whose 25-year-old son Trevor is in the Marines.
Dennis practices at a hospital in Jacksonville, Fla., that recently adopted the military’s protocol when giving blood transfusions to patients with massive injuries.
“They used to learn from us,” Dennis said of military surgeons. “Now we learn from them.”
Wednesday, August 18, 2010
Combat casualty care conference shows promising research returns
"We want to save the most lives we can, and that's hemorrhage," Hack said during a phone conference Tuesday morning.
Col. Lorne Blackbourne, commander of the U.S. Army Institute of Surgical Research in San Antonio, Texas, said his command presented a paper on Monday showing that half of the service members who died of injuries suffered "potentially survivable" wounds. Eighty percent of those were hemorrhages, Blackbourne said. Of those hemorrhages, 30 percent were in the legs and arms, where a tourniquet can be used to stop bleeding; 20 percent were in the neck, groin, armpit and areas where a tourniquet cannot be used but pressure can be applied to stop bleeding; and 50 percent were in the chest or abdomen, where pressure cannot stop the bleeding.
Blackbourne said combat medics are better trained than ever before and the tourniquet technique, in use for about 300 years, is "the most valuable piece of equipment" in the battlefield. He said the more certain way of saving lives is to find ways of bringing blood products to the field though.
In the civilian world, bleeding patients may be pumped with salt water or other clear liquids to keep their blood pressure up until they reach the hospital in a matter of minutes. In a combat zone, on the other hand, bad weather or enemy fire may keep a bleeding service member from being airlifted to a hospital for hours. Without plasma to promote clotting, or a blood transfusion, the patient can bleed to death before reaching the hospital.
Hack said two products showcased at the conference, which is sponsored by the Department of Defense, may be approved for use within a few years. Freeze-dried plasma is already in human trials, and spray-dried plasma is about six months behind, he said.
Wednesday, July 7, 2010
Department of Defense Funding Shows Continued and Bi-Partisan Support for STB® Lifesaving Technologies' Products
The award is to further advance the development of STB®'s Fibrin Adhesive STat (FAST®) dressing, designed to be effective against the full spectrum of blood loss, including severe arterial and venous bleeding. Fifty percent or more combat-related deaths are attributable to uncontrolled hemorrhaging, and with products such as this, thousands of military and civilian lives could be saved.
"We are most appreciative of the support we have continued to receive from the Department of Defense and members of both the Senate and House. This funding is not only invaluable; it serves as a strong statement of their belief in our Fibrin Adhesive STat (FAST®) dressing and their commitment to help us bring our products to the battlefield, emergency responders and operating rooms," said STB® CEO, Richard Moscarello.
Funding for the grant was included in the FY2009 U.S. Department of Defense appropriations bill and received bipartisan support from Senators Richard Burr (R-NC), Ben Cardin (D-MD), Barbara Mikulski (D-MD), Charles Schumer (D-NY) and former Senator Elizabeth Dole (R-NC) and Representatives Bob Etheridge (D-NC), David Price (D-NC) and Chris Van Hollen (D-MD).
"We've all been encouraged by the pre-clinical studies, but it's particularly gratifying that support for our science is backed with significant monetary commitments as well," said STB®'s Moscarello.
About STB®
Founded in 2005, STB Lifesaving Technologies® (STB®) is a privately held, pre-clinical stage, biotechnology development company located in Rockville, Maryland. STB® which stands for "stop the bleeding", is focused on developing a comprehensive suite of products to stop serious and life threatening bleeding in trauma and surgical settings. With a strong proprietary position using its all-natural protein technology with five patents and one provisional patent pending, STB®'s products will be indispensable to both the military and civilian medical markets.
Tuesday, June 29, 2010
HemCon Medical Technologies Introduces GuardIVa™
PORTLAND, Ore., and DUBLIN, Ohio, June 23, 2010 — HemCon Medical Technologies Inc. today announced the launch of the HemCon© GuardIVa™ Antimicrobial Hemostatic IV Dressing, the only antimicrobial dressing containing both a hemostatic compound and the antiseptic agent chlorhexidine gluconate (CHG). The company also announced a five-year sole-source distribution agreement with Cardinal Health to provide the new dressing to hospitals and surgery centers in single sterile units and within Cardinal Health Presource® procedure packs.
Because of its proprietary hemostatic compound, GuardIVa can be used in the first 24 hours after placement of IV devices to control bleeding and provide immediate antimicrobial protection to the IV site. GuardIVa is also able to absorb up to 11 times its own weight in fluid, reducing the need for frequent dressing changes.
GuardIVa’s CHG base also provides more effective and sustained antimicrobial activity over a seven-day span than the silver base used in other antimicrobial dressings.1 The use of CHG-based dressings helps protect against micro-organisms such as Methicillin-resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Staphylococcus epidermidis (MRSE).
“GuardIVa’s superior hemostatic and antimicrobial qualities reduce the need for frequent dressing changes and provide a complete and cost-effective solution to help drive patient safety and IV site infection management,” said John W. Morgan, CEO of HemCon Medical Technologies, Inc. “We’re excited to be able to partner with Cardinal Health to extend this complete IV site care solution to its customer base of infection specialists and health care professionals leading the charge for infection control.”
Central Venous Catheters (CVCs) and Peripherally Inserted Central Catheters (PICCs) are among the ideal applications for GuardIVa.
“We know there is a heightened focus on infection prevention, especially as it relates to catheter-related bloodstream infections,” said Debra Schotz, senior vice president of Patient Care at Cardinal Health. “By including GuardIVa in our portfolio in addition to our full line of Presource standard and custom kits, we’re helping our customers comply with recommendations from the Institute for Healthcare Improvement calling for maximal barrier protection and a kit or central line bundle to improve compliance for catheter insertion and maintenance procedures.” 2
For product information, Cardinal Health customers should contact their nursing products or Presource sales representative. Information is also available through Cardinal Health by calling Jaime Simon at (614) 553-4663 for single sterile use or Crystal Humphreys at (614) 553-5263 for in-kit use.
Sunday, September 20, 2009
Hemostasis, Control of Bloodloss - War, EMS, Military,First Resonders, Ambulance
Tuesday, August 11, 2009
EMS Hemostasis Articles
Monday, February 2, 2009
Woundstat halted by US army
Officials were in the process of distributing some 17,000 packets of WoundStat, granules that are poured into wounds when special bandages, tourniquets or other efforts won't work. But a recent study showed that, if used directly on injured blood vessels, the granules may lead to harmful blood clots, officials said Tuesday.
The Army Medical Command will continue its research and work with the manufacturer in hopes of figuring out in the next few months whether to resume use of WoundStat, said Col. Paul Cordts, head of Army health policy and services.
WoundStat manufacturer TraumaCure, Inc., of Bethesda, Md., had no immediate comment.
The product had been approved by the U.S. Food and Drug Administration. It was one of the latest in a series of Army efforts to improve survival rates on the battlefield.
Today, 90 percent of injured troops survive their wounds, the highest rate of any war, Cordts said in an interview. He credited better training of combat medics, better body armor the troops wear and better tactics they use on the battlefield, as well improved bandages, tourniquets and so on.
Defense Department figures show that as of this month, more than 4,800 troops have been killed in Iraq and the global war on terror. The latter category counts casualties mostly from Afghanistan. Some 34,000 troops have been wounded in the wars, where insurgents have made wide use of roadside bombs and other explosives.
Excessive blood loss is the number one killer on the battlefield, and the Army announced in October that it was sending two potential lifesavers ‹ the WoundStat packets and a bandage called Combat Gauze to replace older other products that had been in use at the time.
A committee of Army medics, Navy corpsmen, surgeons and others recommended the Combat Gauze bandage which has an agent that triggers blood clotting should be the first-line treatment for life-threatening hemorrhaging in cases where a tourniquet could not be placed, such as the armpit or groin area.
The WoundStat granules were to be used if the bandage failed to work.
Cordts said the Army put out a message on Dec. 18, directing the temporary halt in use of WoundStat. Though it has arrived at the war zones, officials are unclear on how widely it has been distributed so far. They¹re working to identify any soldiers who got the treatment, study their cases and examine them for any problems with blood clotting, Cordts said.
He said he didn't know whether it had been used on any soldiers and thus had no reports back from the field positive or negative on how effective it might have been.
Cordts said that after an additional few months of study, officials will likely determine whether they should discontinue its use altogether or perhaps redistribute it with warnings for how it is to be used.
Tuesday, September 23, 2008
TraumaCure Receives CE Mark Clearance to Sell WoundStat in Europe
Wednesday, September 17, 2008
KGI Professor Ian Phillips Awarded Defense Grants for Medical Combat Research
"With battlefield injuries, soldiers can bleed to death before medics are able to reach them," said Phillips. "We are working to produce an automatic anti-hemorrhaging system that would allow a wounded soldier's own body to produce a blood-clotting protein, thus giving him or her potentially life-saving minutes until their injuries can be treated."
The anti-hemorrhaging system, known as the Automatic Hemostat Vector, would be given as an injection to soldiers before going into battle. Phillips research is focusing on a molecule that "switches on" a gene to produce a blood clotting protein, Factor VII.
When a blood vessel is broken, the bleeding reduces oxygen. Low oxygen would activate the Hemostat causing Factor VII to be made in the injured tissue. Factor VII seals broken blood vessels, and as oxygen levels are restored, the Hemostat would turn off automatically in the body.
In addition to helping soldiers, the Hemostat has potential for use in civilian surgery and cases of hemophilia and hemorrhagic stroke. Phillips is also working on ways to add a stem cell homing factor gene to the Hemostat system that would cause stem cells to be drawn to the injury from bone marrow and begin healing the wound even while the injured soldier is on the battlefield.
Phillips' research is funded by a sub-grant from the DoD's Defense Threat Reduction Agency and the US Army's Telemedicine and Advanced Technologies Research Center to the University of South Florida. To date, Phillips has received $323,000 from the Defense Department in support of his research.
The Hemostat system will be developed at KGI and tested at the University of South Florida and the US Army Surgical Institute in San Antonio.
Phillips holds a MERIT Award from the National Institutes of Health, the 2002 Christopher Columbus Award for science and technology and the Lucian Award for research on circulatory disease.
Tuesday, September 16, 2008
TraumaCure Receives Health Canada Licensure
TraumaCure also announced an exclusive distributor agreement with Canadian Tactical and Operational Medical Solutions Inc. (CTOMS), the highly regarded provider of Combat Casualty Care training and supplier of high quality medical equipment, to make WoundStat immediately available throughout Canada. CTOMS is based in Edmonton, Alberta

