PITTSBURGH, Jan. 6, 2015 /PRNewswire/ -- Cohera Medical, Inc.®, a leading innovator and developer of absorbable surgical adhesives and sealants, announced today that it has received a letter from the U.S. Food and Drug Administration (FDA) that the pending Premarket Approval Application (PMA) for its TissuGlu® Surgical Adhesive is Approvable. The receipt of the Approvable Letter means that the FDA has largely approved all parts of the PMA. The Company expects to work with the FDA to reach a final approval on the application within a few weeks.
"The receipt of the PMA Approvable Letter is a truly significant milestone for the Company," said Patrick Daly, Cohera Medical President and CEO. "We are excited about the pending approval for TissuGlu and are looking forward to making TissuGlu available to surgeons and patients throughout the United States."
TissuGlu is indicated for the approximation of tissue layers where subcutaneous dead space exists between tissue planes in abdominoplasty. Upon approval, TissuGlu will be the first internal surgical adhesive of its kind approved in the United States.
Mr. Daly will be presenting on this latest milestone and other significant updates at the J.P. Morgan Healthcare Conference on January 13th at 4:00pm PST.
TissuGlu is designed to meet a surgeon's need for a strong, biocompatible, and easy-to-use surgical adhesive. The Approvable PMA supports the safety and effectiveness of TissuGlu as an alternative to the use of closed suction drains in abdominoplasty procedures, reducing the number of post-operative invasive treatments and improving the patient recovery process.
"TissuGlu, which has the potential to eliminate wound drainage issues from abdominoplasty procedures, represents a major advance in helping patients recover faster and more comfortably following these procedures," said Joseph P. Hunstad MD, FACS, of The Hunstad-Kortesis Center, Charlotte, N.C. "When approved, it will bring great benefits to both patients and surgeons."
"Having the PMA approved, minus a few small labeling changes, signifies the FDA has approved the years of scientific, engineering, manufacturing, quality, and clinical work conducted by the Cohera Medical team," said Chad Coberly, JD, Vice President of Clinical, Regulatory & Legal Affairs for Cohera.
TissuGlu is targeted for use in abdominoplasty procedures in which drains are used to control fluid output and seroma formation. There are approximately 175,000 US-based abdominoplasty procedures per year, growing at an annual rate of 7.7 percent that could utilize TissuGlu. The product has been on the market in the EU since 2011, and currently more than 1,500 procedures have been conducted with TissuGlu bringing great benefit to patients, surgeons, and caregivers.
About Cohera Medical Cohera Medical, Inc. is a rapidly growing medical device company that is actively developing a line of surgical adhesives and sealants. Cohera Medical's products are based on a unique chemical design that is resorbable, non-toxic, easy-to-use, and forms a strong bond between tissue layers. The Company's lead product, TissuGlu® Surgical Adhesive, is indicated in the EU for the approximation of tissue layers where subcutaneous dead space exists between the tissue planes in large flap surgical procedures such as abdominoplasty. TissuGlu is currently approved for sale in the EU and is being utilized to eliminate drains or reduce complications in patients undergoing large flap surgical procedures such as abdominoplasty (tummy tuck), mastectomy, lymph node dissection, decubitus and latissimus dorsi flap procedures. The Company's second product, Sylys® Surgical Sealant, the first synthetic sealant designed specifically to help reduce anastomotic leaks, is currently under the CE Mark approval process. TissuGlu and Sylys are the first in a pipeline of technology that includes surgical mesh adhesives, hemostatic foam, bone adhesives, and drug delivery. Outside of the EU, TissuGlu and the other Cohera Medical products are currently indicated for investigational use only and have not yet been approved for sale by the Food and Drug Administration (FDA) in the U.S. or in any other market.
Showing posts with label adhesive. Show all posts
Showing posts with label adhesive. Show all posts
Tuesday, January 6, 2015
Cohera Medical, Inc.® Receives PMA Approvable Letter from U.S. FDA for TissuGlu® Surgical Adhesive
Labels:
adhesive,
Cohera Medical,
sealant,
TissuGlu
Tuesday, March 2, 2010
Caddisfly larvae spun into surgical tape

SALT LAKE CITY – Often used as fishermen's bait, sticky caddisfly larvae may soon be used to suture wounds, according to researchers at the University of Utah.
The small, moth-like insect spins silk, much like butterflies and spiders, albeit underwater instead of on dry land. The chemical and structural properties of the larvae make it a probable and valuable adhesive tape during surgery because it could be used to hold together skin from the inside.
"I picture it as sort of a wet Band-Aid, maybe used internally in surgery – like using a piece of tape to close an incision, as opposed to sutures," said Russell Stewart, an associate professor of bioengineering at the U. and principal author of a new study of the properties of the fly's silk. The study will be published this week in Biomacromolecules, a journal of the American Chemical Society. He said gluing things together underwater isn't easy.
"Have you ever tried to put a Band-Aid on in the shower? This insect has been doing this for 150 million to 200 million years," he saidStewart studies natural adhesives at the U., including another he discovered made by sandcastle worms on the shore between high tide and low tide ocean waves. That type of natural "glue" has the potential to help repair small broken bones in humans by holding them together. He learned of the potential of the caddisfly larva from a Smithsonian Institution scientist who showed him several of the tube-shaped larval cases that caddisflies spin underwater. It was then that he put on his boots and waded through the Provo River, looking for larvae.
After growing them in his lab, researchers analyzed the silk fibers, finding that they stitched together glass beads from inside their shelters.
"It's like using Scotch tape on the inside of a box to hold it together," Stewart said. "It's really like a tape more than anything else – a tape that works underwater." Next up, they plan to study how strong the silk, or spun larva, can be and whether it can be reproduced synthetically and then used as a surgical adhesive.
In addition to caddisflies, the sandcastle worms, as well as mussels and sea cucumbers, are among the four categories of living organisms that have the ability to make adhesives under water.
And just as it took researchers a while to figure it out, the system of spinning something sticky enough to hold onto its eggs in the aquatic environment evolved independently for the flies, too, helping the creatures live and thrive, Stewart said.
Labels:
adhesive
Sunday, August 2, 2009
Scar-free surgery with nanotechnology sealant
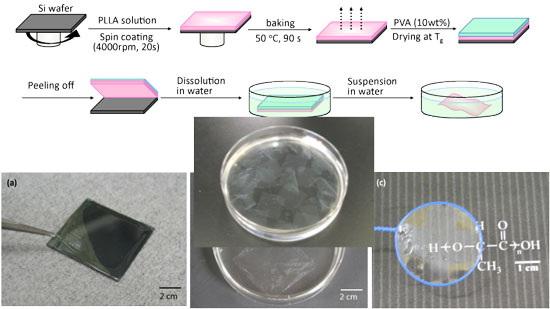
(Nanowerk Spotlight) Much attention of nanotechnology researchers has recently been paid to the fabrication of free-standing, ultra-thin films. These systems have been developed for use in a wide variety of fields such as nano-separation membranes or nanosensors for electrochemical and photochemical applications. In a first report on the fabrication of free-standing nanosheets for biomedical applications, scientists in Japan have developed a biodegradable thin film of only about 20 nanometers thickness that could replace surgical stitches.
"We have developed a free-standing PLLA nanosheet with a thickness of 20nm and an area in centimeter order, fabricated by a simple combination of a spin-coating method and a peeling technique with PVA as a supporting film,"
Shinji Takeoka tells Nanowerk. "We found that our ultra-thin nanosheet has an excellent sealing efficacy for gastric incision as a novel wound dressing that does not require adhesive agents. Furthermore, the sealing operation repaired the incision completely without scars and tissue adhesion. This approach would constitute an ideal candidate for an alternative to conventional suture/ligation procedures, from the perspective not only of a minimally invasive surgical technique but also reduction of operation times."Takeoka is a professor in the Department of Department of Life Science and Medical Bioscience at Waseda University in Tokyo. With collaborators from the National Defense Medical College and members of his group he reports his findings in a recent paper in "Free-Standing Biodegradable Poly(lactic acid) Nanosheet for Sealing Operations in Surgery"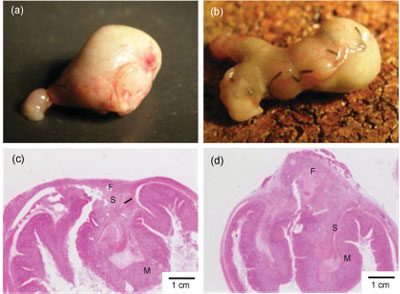

t
Labels:
adhesive,
nano-technology,
sealant
Tuesday, February 3, 2009
Adhesion Barrier Market to Skyrocket to Over $550 Million by 2013
WALTHAM, Mass., Feb 02, 2009 /PRNewswire via COMTEX/ -- Growing interest and innovation will drive adhesion barrier market, according to Millennium Research Group
According to Millennium Research Group's (MRG's) US Markets for Surgical Hemostats, Internal Tissue Sealants, and Adhesion Barriers 2009 report, growing surgeon interest in adhesion barriers, combined with numerous upcoming product launches, will fuel revenues in the US adhesion barrier market. Exceeding $550 million by 2013, the adhesion barrier market will experience a compound annual growth rate of almost 25% over the next five years.
It is estimated that the cost of treating adhesiolysis (the surgical removal of adhesions) in the US is over $2 billion annually. Due in part to the narrow range of approved indications for currently available products, the US adhesion barrier market remains underpenetrated; however, interest in these products is expanding rapidly as hospitals and surgeons realize the many benefits of using an adhesion barrier in surgical procedures. This interest will prompt a growing number of competitors to enter the US adhesion barrier market in the coming years.
The arrival of new adhesion barriers will expand indications, and the associated favorable clinical data that supplement product approvals, will boost surgeon awareness and drive adoption. Improved awareness will further be bolstered by continuing innovation and intensifying competition from market competitors, contributing to unit growth and adhesion barrier market expansion over the next five years.
"Following the removal of Gliatech's ADCON-L, FzioMed's Oxiplex/SP adhesion barrier was slated to be the first such product approved for spinal applications in the US," says Kevin Flewwelling, Manager of Orthopedics at MRG. "But, due to the product's unfavorable approval vote from the FDA's Orthopaedic & Rehabilitative Devices Panel in July of 2008, several companies have demonstrated renewed interest in developing an adhesion barrier approved for use in the lucrative US spine market."
MRG's US Markets for Surgical Hemostats, Internal Tissue Sealants, and Adhesion Barriers 2009 report provides in-depth coverage of the surgical hemostat, internal tissue sealant, and adhesion barrier markets. Competitors covered include Baxter BioSurgery, Covidien, CryoLife, Davol (a subsidiary of C.R. Bard), Ethicon (a Johnson & Johnson company), Genzyme Biosurgery, King Pharmaceuticals, MAST Biosurgery, Orthovita, Pfizer, and many more.
According to Millennium Research Group's (MRG's) US Markets for Surgical Hemostats, Internal Tissue Sealants, and Adhesion Barriers 2009 report, growing surgeon interest in adhesion barriers, combined with numerous upcoming product launches, will fuel revenues in the US adhesion barrier market. Exceeding $550 million by 2013, the adhesion barrier market will experience a compound annual growth rate of almost 25% over the next five years.
It is estimated that the cost of treating adhesiolysis (the surgical removal of adhesions) in the US is over $2 billion annually. Due in part to the narrow range of approved indications for currently available products, the US adhesion barrier market remains underpenetrated; however, interest in these products is expanding rapidly as hospitals and surgeons realize the many benefits of using an adhesion barrier in surgical procedures. This interest will prompt a growing number of competitors to enter the US adhesion barrier market in the coming years.
The arrival of new adhesion barriers will expand indications, and the associated favorable clinical data that supplement product approvals, will boost surgeon awareness and drive adoption. Improved awareness will further be bolstered by continuing innovation and intensifying competition from market competitors, contributing to unit growth and adhesion barrier market expansion over the next five years.
"Following the removal of Gliatech's ADCON-L, FzioMed's Oxiplex/SP adhesion barrier was slated to be the first such product approved for spinal applications in the US," says Kevin Flewwelling, Manager of Orthopedics at MRG. "But, due to the product's unfavorable approval vote from the FDA's Orthopaedic & Rehabilitative Devices Panel in July of 2008, several companies have demonstrated renewed interest in developing an adhesion barrier approved for use in the lucrative US spine market."
MRG's US Markets for Surgical Hemostats, Internal Tissue Sealants, and Adhesion Barriers 2009 report provides in-depth coverage of the surgical hemostat, internal tissue sealant, and adhesion barrier markets. Competitors covered include Baxter BioSurgery, Covidien, CryoLife, Davol (a subsidiary of C.R. Bard), Ethicon (a Johnson & Johnson company), Genzyme Biosurgery, King Pharmaceuticals, MAST Biosurgery, Orthovita, Pfizer, and many more.
Labels:
adhesive,
hemostat,
Market Size,
sealant
Wednesday, July 16, 2008
Cohera get grant for Tissuglu

Cohera Medical Inc. today announced that it has been selected to receive a Phase II Small Business Innovation Research (SBIR) grant of $1.5 million for the ongoing development of TissuGlu, a novel surgical adhesive for application in plastic surgery.
The Phase II SBIR grant, awarded by the National Institutes of Health (NIH), will fund work to finalize the pre-clinical testing that will support submission of an Investigational Device Exemption (IDE) to the Food and Drug Administration (FDA) for approval to initiate human clinical trials. In the second year of the award, human clinical trials will be initiated to establish the product's safety and efficacy.
"Cohera is honored to receive this prestigious and selective award in the current highly competitive environment," said Patrick Daly, president and CEO of Cohera Medical. "Through this award, the NIH acknowledges the innovative nature of Cohera's technology as well as the robust commercial potential of TissuGlu. Our ongoing plans for TissuGlu seek to establish its safety and efficacy as a surgical adhesive to reduce fluid accumulation and the need for drains after surgery. The grant follows the successful completion of the Phase I SBIR project, which established the efficacy of the product formulation and the dispenser device design in preliminary studies."
Labels:
adhesive,
Cohera Medical,
TissuGlu
Monday, June 2, 2008
Haemacure Reports on Financing Progress
MONTREAL, June 2 /CNW Telbec/ - Haemacure Corporation (TSX : HAE), a Montreal-based specialty bio-therapeutics company developing high-value humanplasma-derived protein products for commercialization, provides financing update and reports on second quarter results. Financing As a result of the commitments Haemacure has received to-date from key shareholders to exercise their Series B warrants, the Corporation is wellpositioned to meet its objective of commencing the fibrin sealant pivotalPhase II/Phase III clinical trials. The Corporation remains on schedule and onbudget with the construction of its manufacturing facility. "We are very appreciative and encouraged by the strong  support we have received from our major shareholders, Firebird Management and PinetreeCapital, as demonstrated through the exercise of their Series B warrants."said Joseph Galli, Chairman and CEO of Haemacure. "This support, coupled with the recent addition of Reinaldo Diaz to our Board of Directors and the hiring of senior managers at our facility, are elements of our value creation strategy that will make Haemacure a significant player on the global scale."concluded Mr. Galli.
support we have received from our major shareholders, Firebird Management and PinetreeCapital, as demonstrated through the exercise of their Series B warrants."said Joseph Galli, Chairman and CEO of Haemacure. "This support, coupled with the recent addition of Reinaldo Diaz to our Board of Directors and the hiring of senior managers at our facility, are elements of our value creation strategy that will make Haemacure a significant player on the global scale."concluded Mr. Galli.
Reinaldo M. Diaz
Reinaldo Diaz has over 25 years of experience in the biopharmaceutical industry. Prior to joining Celtic Pharma, he was Managing Member and Co-Founder of D&A Capital Management, LLC (“D&A Capital”), a firm focused on asset management and providing advisory services to companies in the Healthcare sector, particularly biopharmaceutical companies. D&A Capital, through affiliated entities, managed the Delta Opportunity Funds (“Delta”), a group of hedge funds with over $100 million in assets under management.
 support we have received from our major shareholders, Firebird Management and PinetreeCapital, as demonstrated through the exercise of their Series B warrants."said Joseph Galli, Chairman and CEO of Haemacure. "This support, coupled with the recent addition of Reinaldo Diaz to our Board of Directors and the hiring of senior managers at our facility, are elements of our value creation strategy that will make Haemacure a significant player on the global scale."concluded Mr. Galli.
support we have received from our major shareholders, Firebird Management and PinetreeCapital, as demonstrated through the exercise of their Series B warrants."said Joseph Galli, Chairman and CEO of Haemacure. "This support, coupled with the recent addition of Reinaldo Diaz to our Board of Directors and the hiring of senior managers at our facility, are elements of our value creation strategy that will make Haemacure a significant player on the global scale."concluded Mr. Galli.Reinaldo M. Diaz
Reinaldo Diaz has over 25 years of experience in the biopharmaceutical industry. Prior to joining Celtic Pharma, he was Managing Member and Co-Founder of D&A Capital Management, LLC (“D&A Capital”), a firm focused on asset management and providing advisory services to companies in the Healthcare sector, particularly biopharmaceutical companies. D&A Capital, through affiliated entities, managed the Delta Opportunity Funds (“Delta”), a group of hedge funds with over $100 million in assets under management.
Labels:
adhesive,
fibrin,
fibrinogen,
haemacure,
human
Thursday, May 15, 2008
Haemacure Fibrin Market Comparison (featured key slides click to enlarge).
Monday, February 25, 2008
Fibrin Sealants
Most FS products used clinically outside of the U. S. pose certain risks and, as a result, have not been approved by the Food and Drug Administration for use in the U. S. A. For example the FS products available in Europe contain proteins of non-human origin, e. g., aprotinin and bovine thrombin. Consequently, certain individuals are at risk of developing allergic reactions to such non-human protein additives. U. S. Patent No. 6,183, 498 reports that the use of biomedical adhesives have been observed to induce inflammatory tissue reactions.
Both liquefaction processes, however, are associated with significant effort and a considerable time lag before the product can be used in FS products, which can place an already injured patient into a life-threatening situation. Therefore, significant effort has been undertaken to improve the solubility of lyophilized fibrinogen preparations. For example, one manufacturer requires the use of a magnetic stirrer added to the vials of protein to provide significant agitation while heating. This results in dissolution times which are faster than those obtained for the same product without significant mixing, but it still requires 30-60 minutes of preparation time simply to get the fibrinogen ready to use.
Moreover, when heat inactivation is used to inactivate any viruses that may be present in the FS, the process may result in the formation of denatured proteins, which may also be allergenic. For example, the European heat inactivation methods do not inactivate prions which cause bovine spongiform encephalopathy ("mad cow disease"), which has been epidemic recently in bovine herds in European, and hence disease could be carried in the bovine proteins used in the foreign FS products, risking human infection when those products are used for their intended purpose.
Nevertheless, at a sufficiently high fibrinogen concentration, FS preparations provide safe hemostasis, good adherence of the seal to the wound and/or tissue areas, high strength of the adhesions and/or wound sealing, and complete resorbability of the adhesive in the course of the wound healing process (Byrne et al., Br. J. Surg. 78: 841-843 (1991) ). For optimal adhesion, a concentration of fibrinogen of about 15 to 60 mg/ml is required in a ready-to-use tissue adhesive solution (MacPhee, personal communication). The clinical uses of FS products have been reviewed (e. g., by Brennan, Blood Reviews 5: 240-244 (1991) ; Gibble et al., Transfusion 30: 741-747 (1990); Matras, J. Oral Maxillofac. Surg. 43: 605-611 (1985); Lemer et al., J Surg. Res. 48: 165-181 (1990)).
Baxter/Hyland (Los Angeles, Calif.) in conjunction with The American National Red Cross have co-developed Tisseel, the first commercial fibrin sealant to be approved in the United States (see, e. g., U. S. Patent Nos. 6,054, 122; 6,117, 425; and 6,197, 325 (MacPhee et al.). This FS product has advantages over those available in Europe because it is free of bovine proteins. For example, it contains human thrombin, and it contains no aprotinin, thereby reducing the potential for allergenicity. In addition, it is virally inactivated by a solvent detergent method, which produces fewer allergenic denatured proteins.
However, not only does the need to slowly liquefy the protein components cause a significant delay in the formation of the FS preparation, a significant problem arises once fibrinogen is solubilized because its instability results in a tendency to prematurely self- coagulate. In fact, once prepared, the Baxter instructions indicate that the reconstituted solutions can be kept in their respective vials or syringes for a maximum of only 4 hours, after which any unused sealant must be discarded. As a result, the Baxter FS cannot be stored in a ready-to-use condition for any useful length of time.
Both liquefaction processes, however, are associated with significant effort and a considerable time lag before the product can be used in FS products, which can place an already injured patient into a life-threatening situation. Therefore, significant effort has been undertaken to improve the solubility of lyophilized fibrinogen preparations. For example, one manufacturer requires the use of a magnetic stirrer added to the vials of protein to provide significant agitation while heating. This results in dissolution times which are faster than those obtained for the same product without significant mixing, but it still requires 30-60 minutes of preparation time simply to get the fibrinogen ready to use.
Moreover, when heat inactivation is used to inactivate any viruses that may be present in the FS, the process may result in the formation of denatured proteins, which may also be allergenic. For example, the European heat inactivation methods do not inactivate prions which cause bovine spongiform encephalopathy ("mad cow disease"), which has been epidemic recently in bovine herds in European, and hence disease could be carried in the bovine proteins used in the foreign FS products, risking human infection when those products are used for their intended purpose.
Nevertheless, at a sufficiently high fibrinogen concentration, FS preparations provide safe hemostasis, good adherence of the seal to the wound and/or tissue areas, high strength of the adhesions and/or wound sealing, and complete resorbability of the adhesive in the course of the wound healing process (Byrne et al., Br. J. Surg. 78: 841-843 (1991) ). For optimal adhesion, a concentration of fibrinogen of about 15 to 60 mg/ml is required in a ready-to-use tissue adhesive solution (MacPhee, personal communication). The clinical uses of FS products have been reviewed (e. g., by Brennan, Blood Reviews 5: 240-244 (1991) ; Gibble et al., Transfusion 30: 741-747 (1990); Matras, J. Oral Maxillofac. Surg. 43: 605-611 (1985); Lemer et al., J Surg. Res. 48: 165-181 (1990)).
Baxter/Hyland (Los Angeles, Calif.) in conjunction with The American National Red Cross have co-developed Tisseel, the first commercial fibrin sealant to be approved in the United States (see, e. g., U. S. Patent Nos. 6,054, 122; 6,117, 425; and 6,197, 325 (MacPhee et al.). This FS product has advantages over those available in Europe because it is free of bovine proteins. For example, it contains human thrombin, and it contains no aprotinin, thereby reducing the potential for allergenicity. In addition, it is virally inactivated by a solvent detergent method, which produces fewer allergenic denatured proteins.
However, not only does the need to slowly liquefy the protein components cause a significant delay in the formation of the FS preparation, a significant problem arises once fibrinogen is solubilized because its instability results in a tendency to prematurely self- coagulate. In fact, once prepared, the Baxter instructions indicate that the reconstituted solutions can be kept in their respective vials or syringes for a maximum of only 4 hours, after which any unused sealant must be discarded. As a result, the Baxter FS cannot be stored in a ready-to-use condition for any useful length of time.
Baxters Floseal - bovine and human components
Click here for the Instructions Warnings for Baxters Floseal It's a Pdf so you'll need Adobe reader. The video below shows preparation of floseal.
Subscribe to:
Posts (Atom)



